17 Overview: Breast
- Describe the anatomy, structures, and landmarks of the breast tissue
- Describe malignancies of the breast
- Describe the simulation process
- Identify commonly used positioning and immobilization devices used for breast treatments
- Define scan parameters and reference isocenter location for breast simulations
- Discuss special considerations in breast patient positioning
- Define the treatment borders and how they relate to tumor spread
- Describe tumor volumes and margins of breast tumors
- Discuss the various treatment procedures for breast malignancies
- Perform tasks associated with the simulation and treatment of breast malignancies
Key Terms
- Brachytherapy
- Breast-Conserving Therapy
- BRCA 1 & 2
- Chest wall
- Deep Inspiration Breath Hold (DIBH)
- Fluoroscopy
- Hypofractionation
- Inflammatory Breast Cancer
- Ductal Carcinoma In Situ
- Intra-operative Radiation Therapy (IORT)
- Invasive Ductal Carcinoma (IDC)
- Lumpectomy
- Lymphovascular Invasion
- Mammographically Occult Breast Cancer
- Mastectomy
- Mepitel Film
- Partial Breast Irradiation (PBI)
- Posterior Axillary Boost (PAB)
- Stairstep technique
- Supraclavicular fossa
- Surface-Guided Radiation Therapy (SGRT)
- Tangential Fields (tangents)
- Triple-Negative Breast Cancer
Overview: Breast
Breast cancer is the most common cancer diagnosed in women in the US; one out of eight women is projected to develop breast cancer in their lifetime. The average age of diagnosis is 62, and major risk factors include a familial history of breast cancer and BRCA 1 & 2 gene mutations. Approximately 1% of breast cancer diagnosis occur in men.
Breast tissues are located between the second and sixth ribs in the sagittal plane, extending from the suprasternal notch to the xiphoid process. Breast tissue extends from the axilla along the inferolateral edge of the pectoralis major muscle forming the tail of Spence. Two-thirds of breast tissue is found in the deep pectoral fascia that overlies the pectoralis major muscle while the other third rests on the fascia covering the serratus anterior muscle. The breast parenchyma consists of 15 to 20 glandular lobules embedded in adipose and fibrous connective tissue. Each lobe is drained by a duct that opens at the nipple.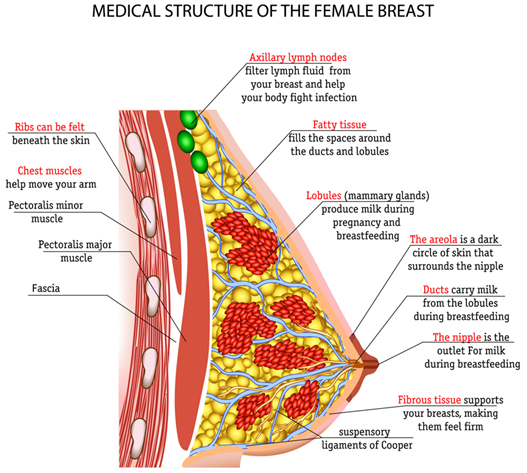
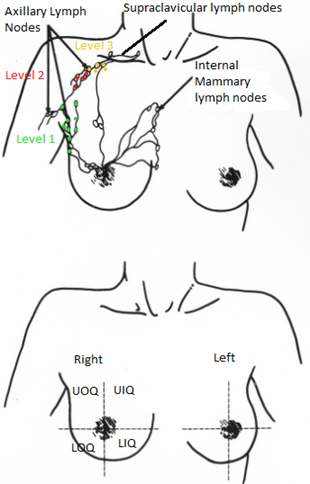
The breast’s lymphatic drainage occurs through three main routes – the axillary lymph nodes, the internal mammary, and the supraclavicular lymph nodes. Most lymphatic drainage occurs via the axillary lymph nodes; this chain is divided into levels I, II, & III. Level I nodes are located lateral of the pectoralis minor muscle and are the most superficial. Level II nodes are located under the pectoralis minor muscle. The level III nodes are found superior and medial to the pectoralis minor muscle and are the most challenging to treat due to their depth. The internal mammary lymph nodes are in the parasternal and intercostal spaces and run alongside the corresponding artery and vein; they are found in the first, second, and third intercostal spaces. The supraclavicular lymph nodes are in the supraclavicular fossa, which provides drainage for internal mammary and axillary lymph node chains. Centrally located tumors may have direct drainage to these nodes requiring inclusion in the treatment.
Breast cancer is classified by its histology and location. The breast is divided into four quadrants; upper outer, upper inner, lower outer, and lower inner. The upper outer quadrant is the most common site of breast cancer. Most breast cancers originate in the milk ducts and lobules. Ductal carcinoma originates in the lactiferous ducts, while lobular carcinoma originates in the breast lobules.
The American Joint Committee on Cancer (AJCC) classifies breast cancers as in situ or invasive.
- Ductal carcinoma in situ (DCIS) is confined to a preexisting duct system of the breast without penetration of the basement membrane and invasion of surrounding tissues on microscopic examination; it represents 21% of all breast cancers. DCIS is a precancerous condition but is treated like cancer.
- Invasive ductal carcinoma (IDC) is the most common invasive cancer of the breast, accounting for 70 to 80% of invasive lesions. This type of breast cancer is also subdivided into grades based on its histology, with well-differentiated tumors assigned a lower grade and poorly differentiated tumors receiving a higher grade.
- Lobular carcinoma in situ (LCIS) is found in the lobules and is like DCIS because it has not demonstrated its ability to penetrate the basement membrane and spread; however, LCIS rarely turns into invasive cancer and typically uses a “watch-and-wait” treatment approach.
- Invasive lobular carcinoma is the second most common type of invasive breast cancer, accounting for 5 to 10% of all invasive lesions.
- Inflammatory breast cancer is an aggressive histology that is more likely to spread before diagnosis. The rapidly proliferating cancer blocks lymph vessels, causing skin edema and pitting, resulting in a peau d’orange (orange peel) appearance. Inflammatory breast cancer is rare, accounting for 1-5% of all breast cancers in the U.S. Patients are classified as Stage III or Stage IV if mets are present.
Breast radiation therapy is done with curative treatment; patients are often diagnosed before the cancer spreads to other parts of the body. Surgery is typically performed as the initial treatment. A mastectomy is the surgical removal of all breast tissue; a lumpectomy is a removal of the tumor plus a margin of breast tissue and is sometimes referred to as breast-conserving therapy. Breast cancer commonly spreads through lymph nodes to the bones, lungs, and liver. Patients that present with metastatic disease may receive palliative radiation to metastasis, but not their breast.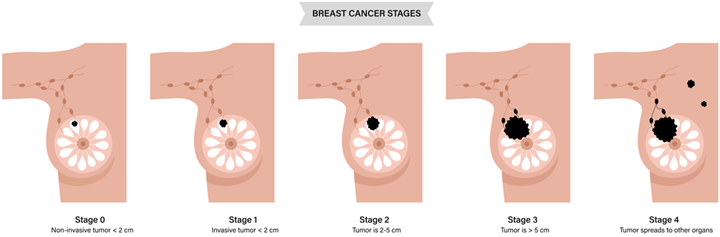
Cell receptor status is an important prognostic factor in breast cancer. Estrogen (ER), Progesterone (PR), and human epidermal growth factor type 2 (HER-2 neu) are proteins of the surface of breast cancer cells that, if present, can promote cancer growth. Targeting these receptors can slow down or prevent cancer growth. For example, estrogen receptor-positive (ER+) tumors exhibit a 5 to 10% lower risk of recurrence than patients with receptor-negative (ER-) cancer. Triple-negative cancers (ER-, PR-, HER2-) lack expression of all receptors and often behave more aggressively than other breast cancers. Targeting these molecular pathways and combining them with other modalities, like surgery and radiation, improves treatment outcomes.
| Weak Risk Factors | Moderate Risk Factors | Strong Risk Factors |
| Family history post menopausal
Late menopause / early menarche Nulliparity Obesity Alcohol Consumption Hormone Replacement Therapy High socioeconomic status |
Older Age
Family history of pre menopausal Personal history of benign conditions Dense breast tissue (>50%)
|
Family history in 3+ relatives or bilateral breast cancer in a premenopausal relative
Evidence of BRCA1/2 mutation Personal history of LCI or benign conditions Dense breast tissue (>75%) |
Patient Simulation: Breast
The simulation procedure commonly used for fractionated breast treatments includes a CT simulation without contrast. Patients will disrobe above the waist and remove jewelry such as necklaces and earrings. Patients are typically positioned supine with their arms above their head in an immobilization device (I.e., armboard, vacloc, breast board), with a knee bolster under their knees. Therapists should instruct patients to extend their chin upward and turn their head away from the treatment side to reduce the dose to the anterior neck, esophagus, and mandible.
Physicians may prefer to define the treatment field borders and breast tissues using a radiopaque wire during simulation. Mastectomy, lumpectomy, and surgical drain scars may not be visible on the CT and are also wired for localization. Departmental protocols will vary, but these markers help delineate areas on the scan that are useful in the treatment planning process.
The therapist should place a reference isocenter mark on the patient’s lateral skin surface, in a stable location – not too close to the arm or on movable tissue. The anterior reference isocenter should fall around the nipple-line in a stable location. BBs are placed at all 3 reference marks for visualization and triangulation in the planning system.
The general scan parameters will extend from the mastoid tip superiorly to include the entire lung volume inferiorly (or as specified by the physician). The scan is taken with the patient breathing normally.
Special Simulation Considerations: Breast
Breast simulations require many special considerations based on the patient’s body habits, tumor location, and the organs at risk. Details should be included in the physician’s simulation order, but often recommendations and modifications need to be made by the therapists. One example is when treating both breasts, the patient’s head should be neutral with their chin extended. Each patient is unique and may benefit from some additional considerations.
Patients may have difficulty raising their arm due to surgery, reconstruction, and lymph node dissection. The angle of the arm on the affected side depends on the patient’s ability to raise their arm without discomfort, their ability to position the arm in a way that reduces skin folds in the supraclavicular area, and the size of the immobilization device and its ability to move through the CT bore. A custom vacloc or cushion molded into the arm board may be required to support their modified treatment position as comfortably as possible.
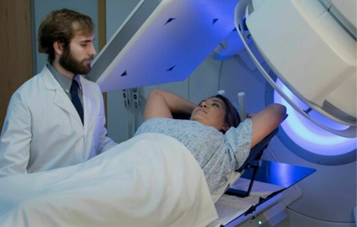
Patients with larger breasts are often positioned on an incline to prevent the breast tissue from being displaced in the infraclavicular area. The incline forces the breast tissue to fall inferiorly, helping to include the entire breast while avoiding dose exposure to the upper arm. Arm extension consistency is important, especially if the Level II, III, and supraclavicular lymph nodes are treated. An index mark documenting the arms extension will help in reproducibility. A bottom-stop will prevent the patient from sliding down during positioning which would affect the couch vertical during treatment.
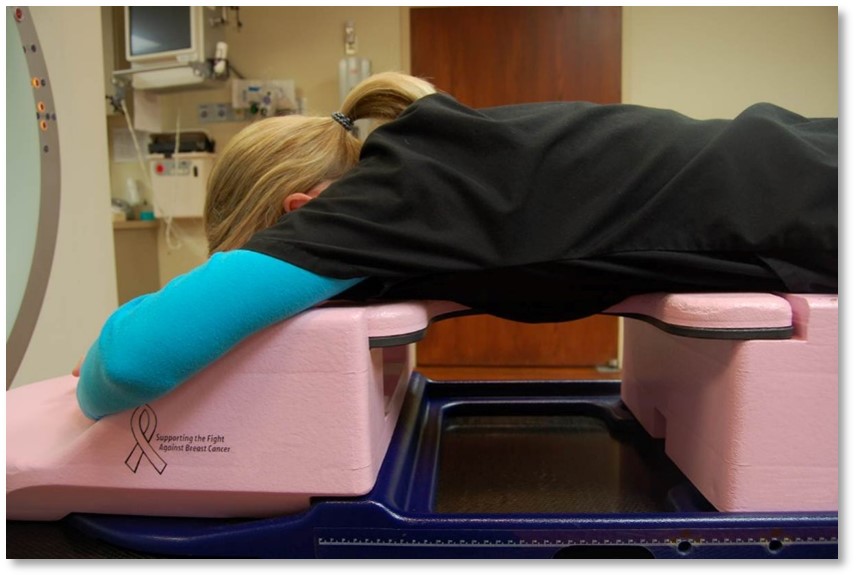
Prone positioning using a breast board is an option for women with large, pendulous, breasts. Advantages to prone positioning include reducing lung tissue in the treatment fields, the gravitational displacement of the breasts away from the body, separation of the contralateral breast from the treatment field, and reduced skin folds and skin reactions.
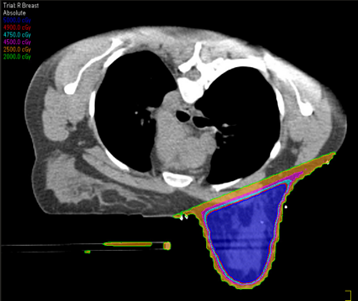
The drawbacks of treating patients in a prone position are it is often uncomfortable and challenging for patients to get into the treatment position – patient selection is important based on their size and ability. The prone position can be difficult for the radiation therapist to reproduce; indexing the patient longitudinally on the board is vital. Additionally, field matching is a challenge because of the limited visualization of the medial border and, therefore, would not be used for a 4-field technique. Sometimes there are clearance issues due to the patient and the device’s vertical height. The heart falls anteriorly towards the treatment field, potentially allowing it to receive a dose.
Breast tissue movement caused by respiration is one of the most differentiating factors from other simulations. Surface-Guided Radiation Therapy (SGRT) using the deep inspiration breath hold (DIBH) or active breathing coordinator (ABC) technique is commonly employed for left-sided breast cancers to move the breast tissue away from the heart. Special circumstances and physician or patient preference may desire to use it for right-sided treatments too.
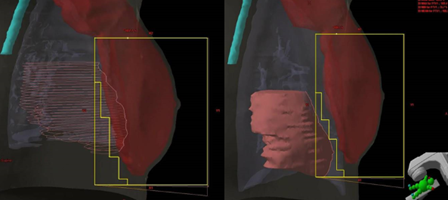
During the simulation process, the patient is evaluated to establish if they are a candidate for the DIBH technique. Patients must be able to hold their breath for the scan’s length – around twenty seconds. A free-breathing scan is also obtained for comparison. Patients breathe normally during the free-breathing scan but are instructed to take a breath and hold it during the inspiration scan. The dosimetrist, medical physicist, and physician will evaluate the scans and the distance of the heart and lungs from the treatment volume to determine the best treatment, DIBH or free-breathing.
Treatment Volume Localization: Breast
The treatment volume, number of fields, and fractionation scheme used will vary. Fields are dependent upon the location of the primary tumor in the breast, the extent of disease, and the amount of lymph node spread. Radiation therapy of the breast typically utilizes lower energy beams, 6-10 MV depending on the depth and amount of breast tissue present. The organs at risk in the treatment of breast cancer are included in the table below.
|
OAR (Organ at Risk)
|
TD 5/5 (Whole Organ)
|
Outcome Associated
|
|
Lung
|
18 Gy
|
Pneumonitis
|
|
Spinal Cord
|
45 Gy or 47 Gy
|
Transverse Myelitis
|
|
Heart
|
40 Gy
|
Pericarditis
|
|
Esophagus
|
55 Gy
|
Stricture
|
|
Brachial Plexus
|
55-60 Gy
|
Clinically apparent nerve damage
|
|
Skin
|
55 Gy
|
Fibrosis
|
Skin care: Patient’s will experience acute effects beginning around 20 Gy with erythema, then dry desquamation around 30 Gy, and moist desquamation at 40+ Gy. Using moisturizing lotions, like Aquaphor, early in the treatment process can reduce side effects and improve comfort. Mepitel Film is used for breast patients to prevent uncomfortable, painful skin reactions from radiation treatments. The dressing-like material prevents tissues from rubbing and sticking together. It does not interact with the beam and can be worn during treatment.
Treatment Fields:
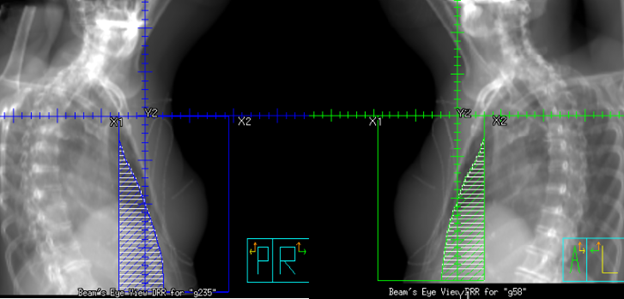
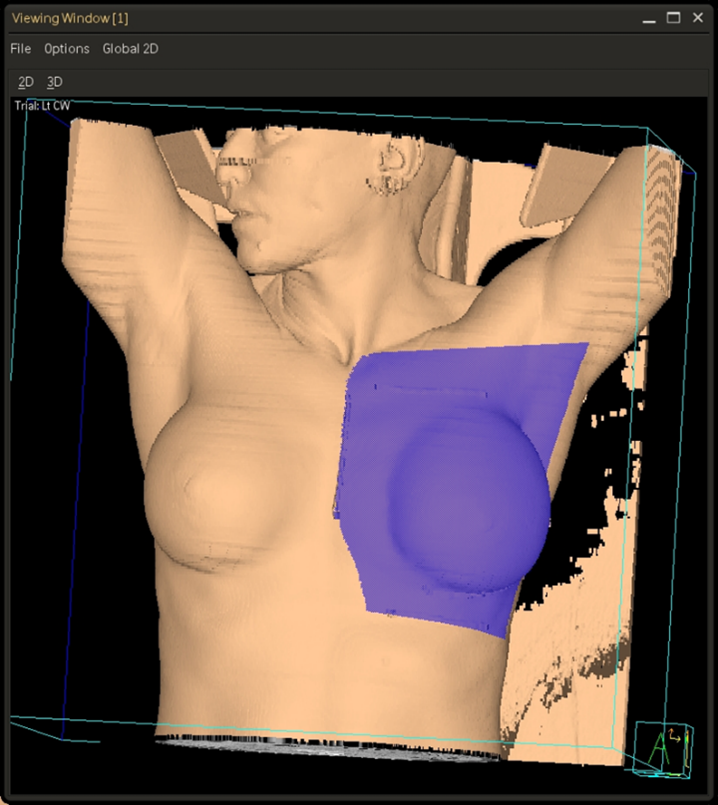
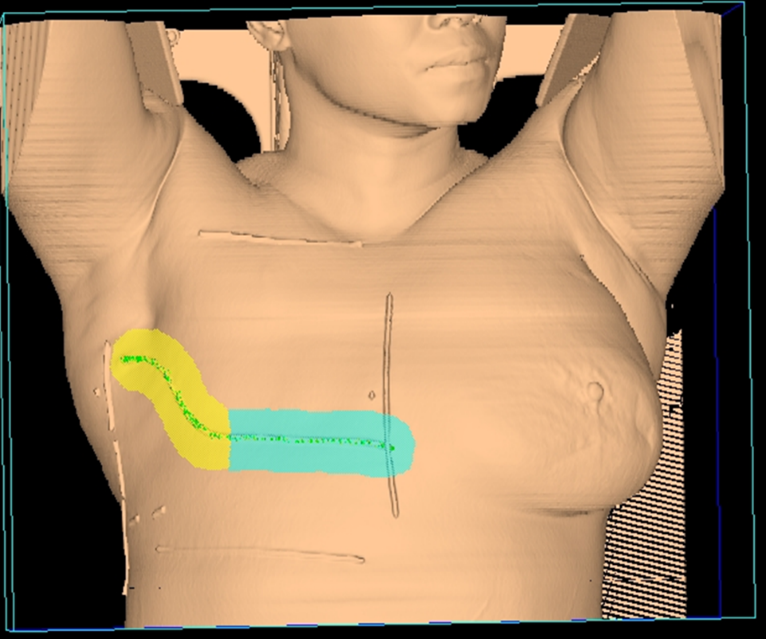
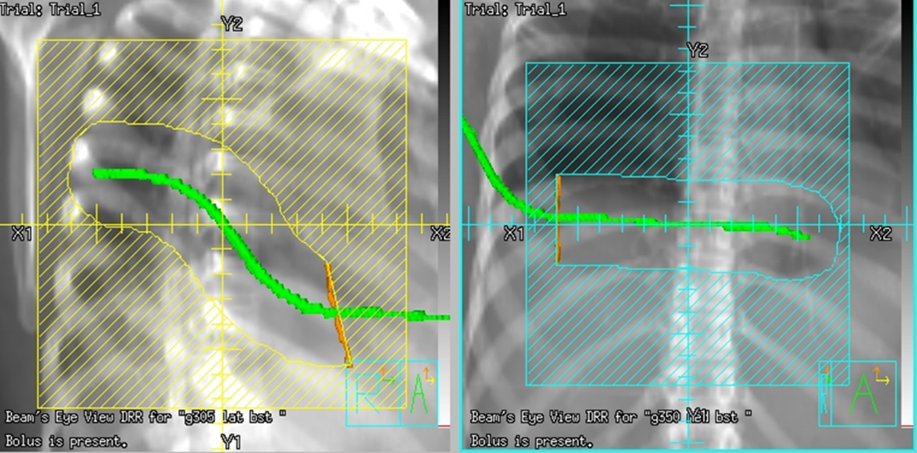
Tangential fields (tangents) are used for early-stage disease and consist of medial and lateral oblique beams. This technique is commonly used to treat the whole breast/chest wall and the Level I axillary lymph nodes while sparing the heart and lungs. The isocenter is centered over the affected breast or chest wall and close to the posterior field border to reduce the beams divergence into the lung. Beams are rotated slightly past 180 degrees to match the beams posterior divergence. Matching the fields is easily accomplished in the treatment planning system or under fluoroscopy using radiopaque wires and the medial and lateral borders clinically.

If treating the chest wall, bolus can be used to bring the dose more superficially to the skin surface. Bolus is a tissue-equivalent density material typically comprised of Paraffin wax, Vaseline gauze, wet gauze or towels, water bags, and other commercially available products. Patients with tissue expanders do not have breast tissue and require bolus for treatment; this presents a unique challenge to form the bolus, free of airgaps, to the patient’s skin surface. Wet towels or a brass chainmail bolus will more easily conform to the patient’s surface. It is important to note, bolus is only used for the tangential fields.
Tangential Treatment borders:
- Medial: Midline (Sternum)
- Lateral: Mid-axillary line (2 cm beyond breast tissue)
- Superior: 1st costal space (or as superior as possible, may be limited by the ipsilateral arm)
- Inferior: 1-2 cm below inframammary fold
- Anterior: Field light fall-off (“flash”) and SSD should be checked during the verification simulation on the linac.
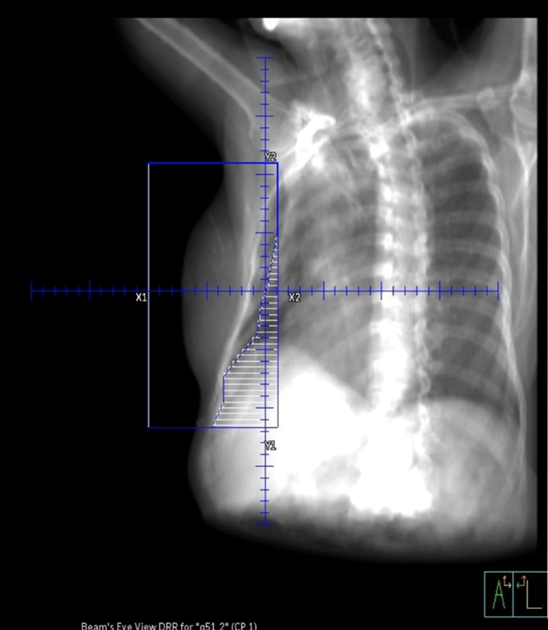
Flash is a visual representation of the area included in the treatment field, and based on breast treatment borders, there should be 1-2 cm of flash over the anterior and inferior surface of the breast. This margin accounts for the respiratory motion to ensure the inclusion of all breast tissue. Place a hand perpendicular to the central ray to check the light field and move it along the patient’s tissue contour to ensure coverage. Source-to-skin distance (SSD) is then verified. The SSD should be within 1 cm of the planned SSD, and the couch vertical referenced from the simulation should also be within 1 cm.
Due to the shape of the breasts and thorax, wedges are used to achieve dose homogeneity and reduce hot spots. The presence or absence of breast tissue determines wedge use. Generally, less breast tissue corresponds to a larger wedge angle required for treatment. For example, a patient who underwent a mastectomy without reconstruction may require a 45-degree wedge for uniform dose distribution. A patient who underwent a lumpectomy with significant breast tissue remaining may only require a 15-degree or no wedge. Some facilities use physical wedges placed in the head of the machine; others use computerized wedges found in the gantry head. Electronic compensation “ec” is another option to achieve dose homogeneity. The multi-leaf collimators (MLCs) are used in a step-and-shoot technique to achieve a uniform dose.
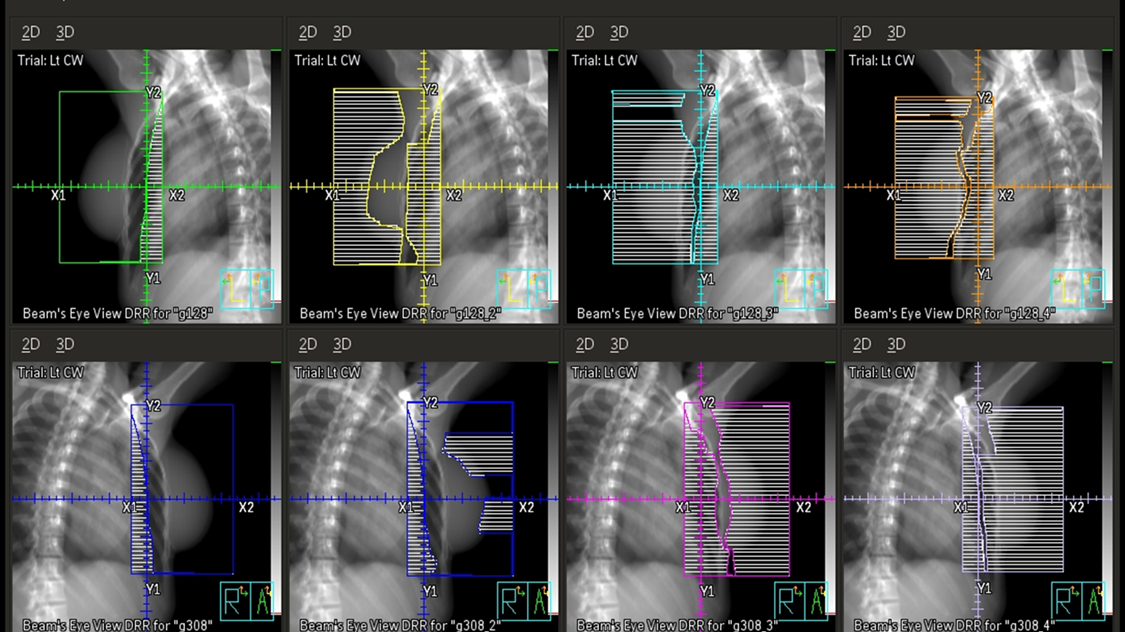
Three & Four-field Techniques
In addition to tangent fields, a third and sometimes fourth field are added when there is nodal involvement. A supraclavicular (supraclav) field is treated for patients with 4+ positive axillary nodes or extracapsular extension; it includes the supraclavicular nodes and the level II & III axillary lymph nodes. Field matching is critical to prevent hot spots or cold spots in the treatment area.
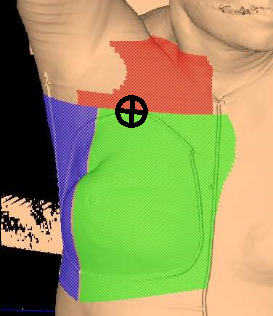
The easiest, most efficient, and safest way to accomplish a field match is using a single isocenter – a monoisocentric technique. This technique utilizes the non-divergent edge of a half-beam block, placing the isocenter at the field match at the level of the sternoclavicular (SC) joint. The lower half of the supraclavicular “supraclav” beam is blocked to avoid divergence into the tangent fields. The superior half of the beam is blocked on the tangential fields. The beam is angled 10-15 degrees mediolaterally (towards the unaffected side) to avoid lateral beam divergence into the spinal cord and esophagus.
For a two isocenter technique, it is important to use a couch kick on the tangential fields. The foot will rotate away from the collimator, so the divergence of cephalad field margins matches the inferior aspect of the supraclav field. This angle is decided using the equation: Tan = half field length/distance. Learn more HERE.
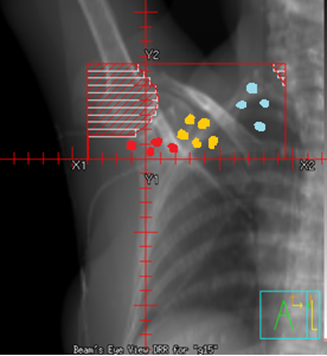
Supraclavicular Treatment borders:
- Medial: 1 cm across midline (or at midline)
- Lateral: From acromioclavicular (AC) joint, bisect humeral head to include coracoid process (Axillary lymph nodes are medial to the humeral head and coracoid process. If undissected axilla, include the entire lateral aspect of the humeral head to include the Level 1 axillary nodes)
- Superior: Approximately 5 cm above suprasternal notch (SSN) extending laterally across neck to the acromion process
- Inferior: 1st costal interspace or abutting the tangent field
Four-field breast treatments encompass the breast tissue/chest wall and the superiorly located lymph nodes that filter from the breast. Fields include tangents, a supraclavicular field, and a posterior axillary boost (PAB) field. The PAB field “boosts” the level III, deep axillary lymph nodes because they receive an insufficient dose from the supraclav field. This beam is usually of higher energy, 10 or 18 MV.
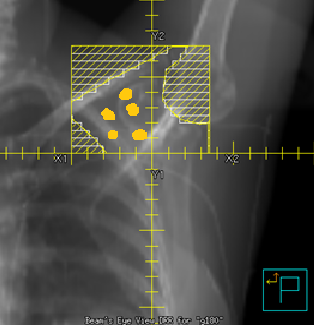
PAB Treatment borders:
- Superior: Bisect clavicle and humeral head
- Inferior: Field matches the superior border of the tangential field (same as supraclav field)
- Medial: Approximately 1 cm of the lung to include the axillary nodes close to the chest wall
- Lateral: Latissimus dorsi muscle
An additional internal mammary field is utilized to include the internal mammary lymph node chain. This field is achieved with a separate electron field. Today, to include these lymph nodes, most centers will extend the medial tangent border past midline (approximately 3cm) to the unaffected side. A radiation therapists should always verify the medial field border with the physician or dosimetrist if the field border is not standard and document it in the patient’s chart.
Treatment Techniques: Breast
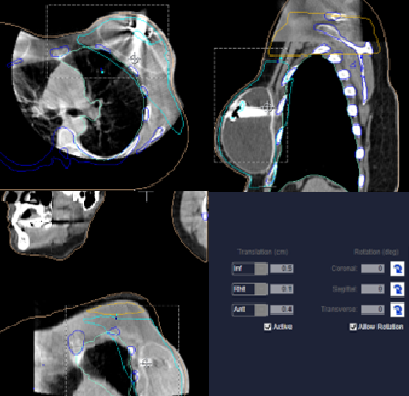
Alignment for breast treatments is typically done through imaging using an orthogonal pair, MV port films, or cone beam CT (CBCT). For VMAT treatments, it is best to align the patient using a CBCT, matching the patient’s body contour, checking the sternum, and humeral head. Because VMAT is very conformal, it is critical to ensure that all the necessary anatomy is included in the imaging and treatment fields. Static fields are standard and not conformal. If using 2D imaging (kV or port films), verify the sternum on the lateral image, and ribs/chest wall on the anterior image. Verify the body, breast tissue, and body contour is reasonable with the tangent port – within falloff margin. If not imaging daily, before each treatment therapists should verify the treatment field falloff anteriorly, check the medial tangent SSD, and reference the couch vertical with the treatment simulation value – values should be within 1 cm.
|
Treatment technique
|
Standard dose fractionation
|
|
Tangents/Chest wall
|
46.8- 50.4 Gy at 1.8-2Gy/fx
|
|
Lymph Nodes: Supraclavicular/PAB
|
45-46.8 Gy at 1.8 Gy/fx
|
|
Tumor bed/scar
|
Boost above doses to ~60 Gy for patients with close margins, recurrent disease, or advanced primary tumors.
|
|
Palliative
|
Bone mets: 8 Gy, 1 fx; 20 Gy, 4 fx; 30 Gy, 10 fx
Fungating Primary: 36 Gy, 6 fx once or twice/week |
In some cases, seromas arise during treatment. Seromas are fluid-filled sacs under the skin’s surface that occur after surgery and during radiation treatment. They can be persistent and must be drained multiple times throughout the radiation course. It is essential to look for these on imaging or the skin, so checking SSD and light fields is essential. If a seroma is found, the patient will likely not be treated that day as they need to see a nurse and doctor to drain the fluid.
Treatment Techniques: Electron Boost
Following the delivery of the initial prescription, patients with close surgical margins, recurrent disease, or advanced primary tumors are “boosted” with an electron or photon treatment to the tumor bed/surgical scar. Boosts are typically set up clinically, though some departments use a dosimetry plan and shift from the primary treatment isocenter to the boost isocenter. Typically, the patient remains in their breast treatment position. The central ray is placed perpendicular (en face) at 100 SSD to the patient’s surface over the treatment area.
Electron fields cannot be imaged; the doctor will approve the patient’s setup before treatment. It’s important to document the treatment field(s) and setup with photos. An option to reproduce the an electron field is to create a template that can be used as a map for future treatments. Using a clear plastic template is beneficial because they do not require putting Tegaderm on a patient’s sensitive skin. Marking the scar(s), moles, birthmarks, or other distinguishing marks on the template is helpful for the reproduction of the treatment field. Label the template with directional landmarks such as medial/lateral, superior/inferior, or anterior/posterior. Patients with a deeper tumor bed or larger breasts sometimes will have a photon boost instead.
Each electron boost patient will have a custom Cerrobend block held in place by an electron cone. The size of cone used is dependent on the block size. The distal edge of the cone, the side closest to the patient, measures 95 cm from the machine source; therefore, the distance from the block to the patient is 5 cm. Safely moving the patient to the appropriate treatment distance is challenging. A stairstep technique is required whenever the gantry is not at 90, 0, or 270 degrees.
Beam energy will vary dependent upon treatment depth; typically, patients are prescribed to the 90% isodose line. The energy required for the 90% isodose line is easily calculated by taking 1/4 the energy. For example, a 12 MeV beam’s 90% isodose line is at a depth of 3 cm; 12/4=3. Sometimes a bolus is required to achieve the desired depth. Department protocol or doctor preference may set the SSD to the bolus or skin surface. For example, if the dose prescribed is to the surface of a 1 cm bolus, the SSD on the skin would read 101 cm; when the bolus is added, the SSD will read 100 cm. The bolus should cover the entire treatment field and be free of air gaps. The physician will determine the prescription and use of bolus; standard boost fractionation schemes consist of a 5-fraction boost with 2 Gy per fraction to 10 Gy.
|
|
| Rp = (practical range) | Energy / 2cm |
| R80 (80% isodose line) | Energy / 2.8 cm (or divide by 3 to ensure coverage) |
| R90 (90% isodose line) | Energy / 3.2 cm (or divide by 4 to ensure coverage) |
Emerging Technologies & Treatments
Breath hold Techniques are most commonly used for left-sided breast cancers.
Hypofractionated treatment schemes are becoming more prevalent. This fractionation style consists of 40 – 42.56 Gy in 15-16 fractions at 2.66 Gy/fx. The benefit of hypofractionation is a decrease in side effects and treatment course length. The tumor bed boost can be treated simultaneously with the primary treatment course. Eligible patients are early stage, low risk, disease.
Partial Breast Irradiation (PBI) is often used to treat early-stage breast cancer patients without nodal involvement; radiation is delivered to the lumpectomy cavity with a margin instead of the whole breast. Patients must meet specific criteria to be candidates for partial breast irradiation. These treatments reduce local recurrence rates and toxicity due to less tissue irradiated, which improves cosmetic outcomes. PBI can be treated via external beam (IMRT/VMAT), intraoperative radiation therapy (IORT), or brachytherapy techniques.
- IMRT/VMAT treatment techniques are becoming the most common technique for partial breast irradiation. This treatment technique has improved cosmesis and provides excellent target coverage to the chest wall and regional lymph nodes with minimal dose to critical structures such as the heart and lungs. This technique also provides a better dose homogeneity. When aligning images, use the surgically placed localization clips in the lumpectomy cavity; if not present, use the body contour and check the sternum. These treatments use a conventional dose-fractionation scheme.
- Intraoperative radiation therapy (IORT) is a single fraction treatment using Intrabeam. Treatment is to the lumpectomy cavity using a 50 kV technique and 10-20 Gy completed during surgery. The tumor cavity has depth requirements to qualify as a candidate for treatment. The University of Iowa’s, Dr. Sugg describes the IORT treatment procedure.
- Brachytherapy high-dose-rate (HDR) procedures commonly use Iridium-192 sources and is referred to as an Accelerated Partial Breast Irradiation (APBI). Treatment involves placing radioactive sources inside or near the tumor site and may be performed for partial breast irradiation. Interstitial brachytherapy treatments are used to treat a quadrant, or less, of the breast. This delivery method requires surgical skill for implantation of a treatment catheter.
- Balloon brachytherapy (Mammosite) is another option that utilizes the placement of a fluid-filled balloon into the breast cavity. The balloon is inserted during the lumpectomy and filled with saline to distend the cavity for treatment. Patient selection requires at least 1 cm of tissue between the skin and the balloon surface. Patients typically receive 10 treatments BID (6 hours between treatments) to 34 Gy. A boost treatment of 10 Gy in 2 fractions over one to two treatment days is prescribed. SAVI is another type of applicator that does not have skin spacing restrictions, making more patients eligible.
Media Attributions
- Breast anatomy © elenbushe is licensed under a All Rights Reserved license
- lymphnodes and quadrants © Unknown adapted by Jared Stiles is licensed under a CC0 (Creative Commons Zero) license
- Breast staging © pikovit is licensed under a All Rights Reserved license
- tang. wires and iso © University of Iowa Hospitals and Clinics Radiation Therapy Program is licensed under a CC BY (Attribution) license
- incline board © Mark Kostich is licensed under a All Rights Reserved license
- prone board © University of Iowa Hospitals and Clinics Radiation Therapy Program is licensed under a CC BY (Attribution) license
- prone plan © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- vert dibh © Vertual Ltd is licensed under a All Rights Reserved license
- 4 field tang © University of Iowa Hospitals and Clinics Radiation Therapy Program is licensed under a CC BY (Attribution) license
- Bolus2.0 © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- 3d lt. tangent © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- drr lt. tangent © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- ec tangent breast © University of Iowa Hospitals and Clinics Radiation Therapy Program is licensed under a CC BY (Attribution) license
- 4 field 3D skin rendering © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- 4 field sclv © University of Iowa Radiation Therapy Program adapted by Jared Stiles is licensed under a CC BY (Attribution) license
- 4 field PAB © University of Iowa Radiation Therapy Program adapted by Jared Stiles is licensed under a CC BY (Attribution) license
- CBCT breast © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- electron skin rendering © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- electron cutouts © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
An internal radiation cancer treatment that implants a radioactive source into the tissue
Usually used for early stage breast cancer to preserve the breast as much as possible without compromising survival (Ex. A lumpectomy followed by radiation therapy as an alternative to a mastectomy)
"Breast Cancer" 1 & 2 gene mutations account for 5-10% of breast cancer diagnosis. People with a BRCA1 mutation have 60-80% lifetime risk of developing breast cancer; BRCA2 results in an increase of 45-70%.
The bony structure surrounding the organs in the thoracic cavity consisting also of skin, fat, and muscles
A radiation technique where the patient holds their breath in order to reduce the amount of dose to the heart
real-time imaging demonstrating motion and/or function
fewer fractions at a higher dose
A rare and aggressive form of breast cancer blocking the lymph vessels in the skin of the breast
An early form of breast cancer that's non-invasive showing the presense of abnormal cells in the milk ducts
Radiation treatment to the tumor bed delivered during surgery
(Aka infiltrating ductal carcinoma) The most common type of breast cancer that starts in the milk ducts and invades into the surrounding tissue
Surgical removal of breast cancer (lump) and surrounding tissue
Cancer within the blood or lymph vessels
Breast cancer not detectable on a mammogram
Surgical removal of the breast
A clear and breathable dressing to protect sensitive skin and prevent skin breakdown
A radiation technique delivered to the tumor bed and surrounding normal tissue instead of the whole breast and is typically used for early-stage breast cancer (aka accelerated partial breast radiation - APBI)
The posterior field used to treat the deep axillary lymph nodes in a 4-field radiation technique. This field can ensure the dose to the mid-axillary area is evenly distributed.
Used when treating electron patients by moving the table half the distance vertically and half laterally to align your treatment light field and SSD
The area indented above the clavicle containing the supraclavicular lymph nodes
A technique using 3D camera systems to accurately align and monitor the patient precisely using the patients' surface
The opposing fields used in the 2-field radiation technique that treat the whole breast and/or chest wall
When the cancer cells don't have estrogen (ER) or progesterone (PR) receptors and don't make much of the HER2 protein, resulting in a worse prognosis

