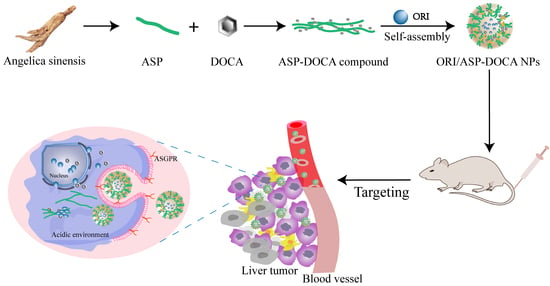
Angelica Sinensis Polysaccharide-Based Nanoparticles for Liver-Targeted Delivery of Oridonin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ASP
2.2. Characterization of ASP-DOCA Compound
2.3. Synthesis and Characterization of ORI/ASP-DOCA NPs
2.4. Cytotoxicity of ORI/ASP-DOCA NPs In Vitro
2.5. In Vitro Liver Targeting Study of ORI/ASP-DOCA NPs
2.6. Cellular Uptake of ORI/ASP-DOCA In Vitro
2.7. Liver Targeting and Antitumor Effect of ORI/ASP-DOCA NPs In Vivo
3. Materials and Methods
3.1. Materials
3.2. Preparation and Physicochemical Characteristics of ASP
3.3. Synthesis and Characterization of ASP-DOCA Compound
3.4. Establishment of Methodology of HPLC Analysis
3.5. Preparation and Characterization of ORI/ASP-DOCA NPs
3.6. In Vitro Drug Release
3.7. Cell Culture
3.8. Cytotoxicity Assay
3.9. In Vitro Cellular Uptake Assay
3.9.1. ORI Cellular Uptake Observed by HPLC
3.9.2. Cellular Uptake Observed by Confocal Laser Scanning Microscope (CLSM)
3.9.3. Cellular Uptake Observed by Flow Cytometry
3.10. In Vitro Liver Targeting Study of ORI/ASP-DOCA NPs
3.11. In Vivo Liver Targeting and Antitumor Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hibi, T. Surgical treatment of hepatocellular carcinoma. Biosci. Trends 2021, 15, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Tomášek, J.; Kiss, I. Hepatocellular carcinoma future treatment options. Klin. Onkol. 2020, 33 (Suppl. S3), 26–29. [Google Scholar] [CrossRef]
- Du, Y.; Liu, D.; Du, Y. Recent advances in hepatocellular carcinoma therapeutic strategies and imaging-guided treatment. J. Drug Target. 2022, 30, 287–301. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Du, D.; Gu, X.; Zhang, X.; Hong, G.; Lai, X. Nanotargeted Cationic Lipid Microbubbles Carrying HSV-TK Gene Inhibit the Development of Subcutaneous Liver Tumor Model After HIFU Ablation. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2024, 43, 95–107. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting—Strategies and applications. J. Control. Release Off. J. Control. Release Soc. 2015, 203, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Monestier, M.; Charbonnier, P.; Gateau, C.; Cuillel, M.; Robert, F.; Lebrun, C.; Mintz, E.; Renaudet, O.; Delangle, P. ASGPR-Mediated Uptake of Multivalent Glycoconjugates for Drug Delivery in Hepatocytes. Chembiochem A Eur. J. Chem. Biol. 2016, 17, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Santo, D.; Cordeiro, R.A.; Mendonça, P.V.; Serra, A.C.; Coelho, J.F.J.; Faneca, H. Glycopolymers Mediate Suicide Gene Therapy in ASGPR-Expressing Hepatocellular Carcinoma Cells in Tandem with Docetaxel. Biomacromolecules 2023, 24, 1274–1286. [Google Scholar] [CrossRef]
- Yamansarov, E.Y.; Lopatukhina, E.V.; Evteev, S.A.; Skvortsov, D.A.; Lopukhov, A.V.; Kovalev, S.V.; Vaneev, A.N.; Shkil, D.O.; Akasov, R.A.; Lobov, A.N.; et al. Discovery of Bivalent GalNAc-Conjugated Betulin as a Potent ASGPR-Directed Agent against Hepatocellular Carcinoma. Bioconjugate Chem. 2021, 32, 763–781. [Google Scholar] [CrossRef]
- Chen, X.P.; Li, W.; Xiao, X.F.; Zhang, L.L.; Liu, C.X. Phytochemical and pharmacological studies on Radix Angelica sinensis. Chin. J. Nat. Med. 2013, 11, 577–587. [Google Scholar] [CrossRef]
- Sabeel, Z.; Liang, Y.; Hao, M.; Ying, L.; Guo, R.; Chen, R.; Li, X.; Yu, C.; Yang, Z. A comprehensive review of antitumor properties of Angelica species and their antitumor-responsible constituents and the underlying molecular mechanisms involved in tumor inhibition. Phytother. Res. PTR 2023, 37, 2187–2211. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.-W.; Sun, R.; Li, X.; Wu, D.; Hu, J.-N. Colon-Targeting Angelica sinensis Polysaccharide Nanoparticles with Dual Responsiveness for Alleviation of Ulcerative Colitis. ACS Appl. Mater. Interfaces 2023, 15, 26298–26315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.; Luo, L.; Cui, Z.; Wang, N.; Shu, Y.; Wang, K.-P. Pharmacokinetics, biodistribution and receptor mediated endocytosis of a natural Angelica sinensis polysaccharide. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 254–263. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, H.; Zhang, X.; Sun, Z.; Wang, F.; Cheng, J.; Xie, H.; Yu, B.; Zhou, L. Deoxycholic acid-modified chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with paclitaxel for enhanced antitumor efficacy. Int. J. Pharm. 2014, 475, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Sadri, F.; Rostami, M.; Mirian, M.; Taymouri, S. Synthesis of pectin-deoxycholic acid conjugate for targeted delivery of anticancer drugs in hepatocellular carcinoma. Int. J. Biol. Macromol. 2019, 139, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Z.; Mei, H.; Xu, J.; Zhou, T.; Cheng, F.; Wang, K. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr. Polym. 2019, 219, 143–154. [Google Scholar] [CrossRef]
- Guo, C.; Hou, X.; Liu, Y.; Zhang, Y.; Xu, H.; Zhao, F.; Chen, D. Novel Chinese Angelica Polysaccharide Biomimetic Nanomedicine to Curcumin Delivery for Hepatocellular Carcinoma Treatment and Immunomodulatory Effect. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 80, 153356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Dai, M.; Nai, J.; Zhu, L.; Sheng, H. Solubility and Bioavailability Enhancement of Oridonin: A Review. Molecules 2020, 25, 332. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhao, J.; Li, Y.; Zhu, L.; Jin, M.; Wang, L.; Liu, J.; Lei, J.; Li, Z. Self-Assembled pH-Sensitive Nanoparticles Based on Ganoderma lucidum Polysaccharide–Methotrexate Conjugates for the Co-delivery of Anti-tumor Drugs. ACS Biomater. Sci. Eng. 2021, 7, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y.; Wang, Q.; Wang, H.; Mei, Q. Analysis of the monosaccharide components in Angelica polysaccharides by high performance liquid chromatography. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2005, 21, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Arif, M.; Feng, C.; Zeenat, S.; Liu, C.G. Synthesis and evaluation of pH-sensitive, self-assembled chitosan-based nanoparticles as efficient doxorubicin carriers. J. Biomater. Appl. 2017, 31, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Fu, R.; Yang, J.; Ma, P.; Liang, L.; Mi, Y.; Fan, D. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int. J. Nanomed. 2019, 14, 6971–6988. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Guan, Y.; Chang, M.; Zhang, F.; Lu, S.; Wei, T.; Shao, W.; Lin, G. RGD(Arg-Gly-Asp) internalized docetaxel-loaded pH sensitive liposomes: Preparation, characterization and antitumor efficacy in vivo and in vitro. Colloids Surf. B Biointerfaces 2016, 147, 90–99. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Food Funct. 2018, 9, 1829–1839. [Google Scholar] [CrossRef]
- Abdou, R.; Mojally, M.; Attia, H.G.; Dawoud, M. Cubic nanoparticles as potential carriers for a natural anticancer drug: Development, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2023, 13, 2463–2474. [Google Scholar] [CrossRef]
- Tavakol, M.; Montazeri, A.; Naghdabadi, R.; Hajipour, M.J.; Zanganeh, S.; Caracciolo, G.; Mahmoudi, M. Disease-related metabolites affect protein-nanoparticle interactions. Nanoscale 2018, 10, 7108–7115. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Ling, X.; Chaurasiya, B.; Yang, C.; Du, Y.; Tu, J.; Xiong, Y.; Sun, C. Efficient delivery of paclitaxel into ASGPR over-expressed cancer cells using reversibly stabilized multifunctional pullulan nanoparticles. Carbohydr. Polym. 2017, 159, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Depalo, N.; Re, F.; Dal Magro, R.; Panniello, A.; Margiotta, N.; Fanizza, E.; Lopalco, A.; Laquintana, V.; Cutrignelli, A.; et al. PEGylated solid lipid nanoparticles for brain delivery of lipophilic kiteplatin Pt(IV) prodrugs: An in vitro study. Int. J. Pharm. 2020, 583, 119351. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Pi, C.; Wen, J.; He, Y.; Yuan, J.; Shen, H.; Zhao, W.; Zeng, M.; Song, X.; Lee, R.J.; et al. Formulation of the novel structure curcumin derivative-loaded solid lipid nanoparticles: Synthesis, optimization, characterization and anti-tumor activity screening in vitro. Drug Deliv. 2022, 29, 2044–2057. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Gu, B.; Paul, M.E.; Wang, B.; Yu, Y.; Feng, Z.; Ma, Y.; Wang, X.; Chen, H. Animal model of intrahepatic metastasis of hepatocellular carcinoma: Establishment and characteristic. Sci. Rep. 2020, 10, 15199. [Google Scholar] [CrossRef]
- Cheng, C.S.; Tan, H.Y.; Zhang, C.; Chan, Y.T.; Zhang, Z.J.; Man, K.; Yuen, M.F.; Wang, N.; Feng, Y. Berberine suppresses metastasis and recurrence of hepatocellular carcinoma by targeting circulating tumour cells: Abridged secondary publication. Hong Kong Med. J. 2022, 28 (Suppl. S6), 10–11. [Google Scholar]
- Zhang, S.; He, B.; Ge, J.; Li, H.; Luo, X.; Zhang, H.; Li, Y.; Zhai, C.; Liu, P.; Liu, X.; et al. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int. J. Biol. Macromol. 2010, 47, 546–550. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, X.; Gao, K.; Liu, W.; Li, N.; Cheng, N.; Ding, G.; Huang, W.; Wang, Z.; Xiao, W. Relationship between molecular weight, monosaccharide composition and immunobiologic activity of Astragalus polysaccharides. Glycoconj. J. 2016, 33, 755–761. [Google Scholar] [CrossRef]
- Xu, D.J.; Xia, Q.; Wang, J.J.; Wang, P.P. Molecular weight and monosaccharide composition of Astragalus polysaccharides. Molecules 2008, 13, 2408–2415. [Google Scholar] [CrossRef]
- Sun, R.; Fang, L.; Lv, X.; Fang, J.; Wang, Y.; Chen, D.; Wang, L.; Chen, J.; Qi, Y.; Tang, Z.; et al. In vitro and in vivo evaluation of self-assembled chitosan nanoparticles selectively overcoming hepatocellular carcinoma via asialoglycoprotein receptor. Drug Deliv. 2021, 28, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, N.; Yan, L.; Gao, F.; Ji, D.; Zhang, S.; Zhang, L.; Li, Y.; Xiao, Y. Preparation, characterisation and in vitro and in vivo evaluation of CD44-targeted chondroitin sulphate-conjugated doxorubicin PLGA nanoparticles. Carbohydr. Polym. 2019, 213, 17–26. [Google Scholar] [CrossRef]
- Li, H.; Yan, L.; Tang, E.K.Y.; Zhang, Z.; Chen, W.; Liu, G.; Mo, J. Synthesis of TPGS/Curcumin Nanoparticles by Thin-Film Hydration and Evaluation of Their Anti-Colon Cancer Efficacy In Vitro and In Vivo. Front. Pharmacol. 2019, 10, 769. [Google Scholar] [CrossRef]
- Kazemi, M.; Emami, J.; Hasanzadeh, F.; Minaiyan, M.; Mirian, M.; Lavasanifar, A.; Mokhtari, M. In Vitro and In Vivo Evaluation of Novel DTX-Loaded Multifunctional Heparin-Based Polymeric Micelles Targeting Folate Receptors and Endosomes. Recent Pat. Anti-Cancer Drug Discov. 2020, 15, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023, 49, 159–173. [Google Scholar] [CrossRef] [PubMed]














| NPs | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| ASP-DOCA | 187.6 ± 3.3 | 0.246 ± 0.015 | −12.38 ± 4.72 |
| ORI/ASP-DOCA | 195.1 ± 3.4 | 0.233 ± 0.018 | −18.96 ± 5.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Nai, J.; Deng, B.; Zheng, Z.; Chen, X.; Zhang, C.; Sheng, H.; Zhu, L. Angelica Sinensis Polysaccharide-Based Nanoparticles for Liver-Targeted Delivery of Oridonin. Molecules 2024, 29, 731. https://doi.org/10.3390/molecules29030731
Sun H, Nai J, Deng B, Zheng Z, Chen X, Zhang C, Sheng H, Zhu L. Angelica Sinensis Polysaccharide-Based Nanoparticles for Liver-Targeted Delivery of Oridonin. Molecules. 2024; 29(3):731. https://doi.org/10.3390/molecules29030731
Chicago/Turabian StyleSun, Henglai, Jijuan Nai, Biqi Deng, Zhen Zheng, Xuemei Chen, Chao Zhang, Huagang Sheng, and Liqiao Zhu. 2024. "Angelica Sinensis Polysaccharide-Based Nanoparticles for Liver-Targeted Delivery of Oridonin" Molecules 29, no. 3: 731. https://doi.org/10.3390/molecules29030731