Abstract
Species from genus Artemisia are widely distributed throughout temperate regions of the northern hemisphere and many cultures have a long-standing traditional use of these plants as herbal remedies, liquors, cosmetics, spices, etc. Nowadays, the discovery of new plant-derived products to be used as food supplements or drugs has been pushed by the exploitation of bioprospection approaches. Often driven by the knowledge derived from the ethnobotanical use of plants, bioprospection explores the existing biodiversity through integration of modern omics techniques with targeted bioactivity assays. In this work we set up a bioprospection plan to investigate the phytochemical diversity and the potential bioactivity of five Artemisia species with recognized ethnobotanical tradition (A. absinthium, A. alba, A. annua, A. verlotiorum and A. vulgaris), growing wild in the natural areas of the Verona province. We characterized the specialized metabolomes of the species (including sesquiterpenoids from the artemisinin biosynthesis pathway) through an LC–MS based untargeted approach and, in order to identify potential bioactive metabolites, we correlated their composition with the in vitro antioxidant activity. We propose as potential bioactive compounds several isomers of caffeoyl and feruloyl quinic acid esters (e.g. dicaffeoylquinic acids, feruloylquinic acids and caffeoylferuloylquinic acids), which strongly characterize the most antioxidant species A. verlotiorum and A. annua. Morevoer, in this study we report for the first time the occurrence of sesquiterpenoids from the artemisinin biosynthesis pathway in the species A. alba.
Similar content being viewed by others
Introduction
Bioprospection is defined as the systematic and organized exploration of natural resources and products derived from microorganisms, plants, fungi and animals, whose exploitation has the potential to generate significant economic and social benefits1. This approach has been applied for centuries by humans, who have always looked at plants as a crucial source of botanical drugs and other compounds for their well-being. Nowadays, about 25% of the drugs prescribed in modern medicine come from plants or are synthetic derivatives obtained from molecular plant precursors2, while, on the other side, a great part of the population living in developing countries still relies on traditional herbal remedies for their primary healthcare (WHO reports). However, recent literature estimates that only 15% of the species that are used for the treatment of diseases (about 70,000) have been studied for their medical use2, emphasizing the imperative for additional exploration. The discovery of new plant-derived drugs has classically relied on ethnobotanical information3, as in the case of artemisinin discovery from the herbaceous plant Artemisia annua4,5. Nonetheless, with the rise of omics sciences, new perspectives have emerged about the combination of ethnobotanical, phytochemical, and molecular phylogenetic data, and promising predictions of medicinal plant uses have been developed3,6. For instance, the improvement of DNA sequencing techniques enabled the phylogenetic reconstruction of plant clusters that include species with a prominent medicinal use and demonstrated bioactivities, allowing the acceleration for the discovery of new potential bioactive compounds in related taxa3.
The Artemisia genus (family Asteraceae) comprises about 500 species of herbs and shrubs that are either biannual, annual or perennial7 and that are widely distributed in temperate regions of Europe, Asia, North Africa and North America. In many cultures, several species from this genus are characterized by a long-standing ethnobotanical use including preparation of herbal remedies for treatment of major and minor ailments (e.g. fever, hypertension, diabetes and malaria), preparation of herbal teas, alcoholic beverages, tonics and cosmetics or cultivation as crops8,9. Many recent studies have unveiled the pleiotropic pharmacological profile of the genus reporting a broad spectrum of bioactivities reflecting the huge number and variety of phytochemicals found in each species. These include anthelmintic, antimalarial, antitubercular, antiviral, antihyperlipidemic, antiemetic, antidepressant, anticancer, anti-asthmatic, antihypertensive, antidiabetic, anxiolytic, hepatoprotective, gastroprotective, and insecticidal action7,10,11,12.
In this work we set up a bioprospection plan based on literature of local flora to investigate the phytochemical diversity and the potential bioactivity of five Artemisia species with recognized ethnobotanical tradition: A. absinthium L., A. alba Turra, A. annua L., A. verlotiorum Lamotte, A. vulgaris L. (Table 1). These species grow spontaneously in the natural areas within the province of Verona and within the Lessinia regional park, an area featured by hilly and mountainous environments, where the climate is classified as cold with no dry season and warm summer13. In this bioprospection work, we combined a sampling plan covering different growing seasons with an untargeted metabolomics approach to profile the specialized metabolomes of the five Artemisia species (including sesquiterpenoids from the artemisinin biosynthesis pathway) and correlate them with the in vitro antioxidant activity measured by FRAP and DPPH assays.
Results and discussion
Metabolic profiles of Artemisia spp. methanolic extracts
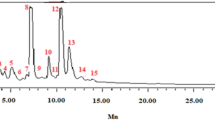
In this work, the high sensitivity and wide analytical range of an LC–MS-based untargeted approach was used to profile nonvolatile medium-polar metabolites extracted from the aerial organs of five Artemisia species (A. absinthium, Aab; A. alba, Aal; A. annua, Aan; A. verlotiorum, Ave; A. vulgaris, Avu) collected throughout three sampling seasons (2019–2021) in the natural areas around Verona (Fig. 1).
Methanolic extracts of Artemisia spp. leaves and stems were produced and analyzed by untargeted UPLC–ESI–MS leading to the detection of 226 m/z features in negative ionization mode (Supplementary File 1) and to the identification of 80 secondary metabolites. The latter are listed in Table 2 together with their chromatographic and spectral features. Representative chromatographic profiles of leaf and stem methanolic extracts are shown in Fig. 2, together with pie charts depicting the metabolome composition according to the percentage of each metabolite class with respect to the total LC–MS signal detected. Moreover, to describe single compounds characterizing the various Artemisia species, a relative comparison of the percentages of all identified metabolites, grouped according to the respective metabolite class, is reported in the heatmap of Fig. 3.
Secondary metabolomes of Artemisia spp. Exemplificative base peak chromatograms recorded in LC–MS–ESI− (intensity scaled to 2.5 × 105) of leaves (left) and stems (right) are shown together with pie charts representing the metabolite classes according to the total LC–MS signal detected. Peak annotation numbers refer to Table 2. HBA hydroxybenzoic acid, HCA hydroxycinnamic acid derivatives.
Heat map showing the average relative percentage abundance (respect to the total LC–MS signal of each metabolome) of all metabolites tentatively identified in Artemisia spp. Leaves (L) and stems (S) over a 3-year sampling period (2019, 2020, 2021). The metabolite identification numbers match those in the dataset. Aab, A. absinthium; Aal, A. alba; Aan, A. annua; Ave, A. verlotiorum; Avu, A. vulgaris. Cf caffeoyl, CfQa caffeoylquinic acids, CfHa caffeoylhexaric acids, HBA hydroxybenzoic acid, HCA hydroxycinnamic acid, der derivatives. This heatmap was created manually from the dataset with the conditional formatting tool of Microsoft Excel (v. 2401; https://www.microsoft.com/it-it/microsoft-365/excel).
The main specialized metabolites composing the metabolomes of Artemisia spp. comprise derivatives of hydroxycinnamic acid (HCA), mainly caffeic and ferulic acid differentially esterified with one or multiple organic acid moieties, O-glycosylated flavonoids (mainly flavonols and flavones together with their O-methylated derivatives), glycosylated coumarins and a few other metabolites identified as glycosides of hydroxybenzoic acids, lignans, saponins and sesquiterpenes. In general, a high level of metabolome characterization was achieved, resulting in a metabolite annotation range of 85–97% of total LC–MS signal, with A. annua and A. abisnthium being the species with the higher proportion of unidentified metabolites left. The HCA derivatives represented the most abundant metabolite class, ranging from 62 to 94% of total LC–MS signal in A. absinthium and A. verlotiorum, respectively. As can be observed in the chromatograms of Fig. 2, in all five species this class was dominated by the caffeic acid esters of quinic acid, in particular two caffeoylquinic acid isomers (compounds 9, 15) and many isomers of dicaffeoylquinic acid (28, 33, 34, 37, 38, 40, 41). The prevalence of caffeic acid derivatives in the Artemisia metabolome is well pronounced in A. absinthium, A. verlotiorum and A. vulgaris, in which they represent the main peaks, whereas a more diversified profile is observed in favor of other compound classes in A.alba and A,annua (e.g., other HCA derivatives, coumarins and flavonoids).
A detailed description of the metabolic profiles of leaves and stems of the five Artemisia species is reported below according to data presented in Figs. 2 and 3.
A. absinthium (Fig. 2A) leaves are mainly characterized by the presence of two isomers (9, 15) of caffeoylquinic acid, one of them identified as 3-O-caffeoylquini acid (chlorogenic acid; 15) and of two abundant isomers of dicaffeoylquinic acid (34, 38); also caffeoylquinic acid hexoside was best represented in this species (7). The leaves of A. absinthium, compared to the other species, present higher proportion of the flavonols kaempferol-O-hexoside-deoxyhexoside (23), isorhamnetin-O-hexoside-deoxyhexoside (30), eupatolitin-O-deoxyhexoside-O-hexoside (32) and isorhamnetin-3-O-glucoside (36). Moreover, in leaves and, at a lower level, in stems of this species only, we detected high amounts of the dimeric guaianolide absinthin (81), which is described as a specific marker of A. absinthium and is responsible for the bitterness of absinth7,28,42. In addition, two other compounds annotated as guaiane-type sesquiterpene dimers (84, 85) were putatively identified according to recent literature data and in silico fragmentation analysis39,40. The stems of A. absinthium showed a much more simplified profile, including the same caffeoylquinic acid isomers found in the leaves together with two less represented dicaffeoylquinic acid isomers (40, 55). Furthermore, in both leaves and stems we observed two unidentified metabolites with a molecular ion of 189.07 m/z (11) and 571.25 m/z (83) characterized, respectively, by higher and lower polarity.
Leaves and stems of A. alba (Fig. 2B) were strongly characterized by the glycosides of different coumarins including esculetin hexoside (6), coumarin-O-hexoside (8), methoxycoumarin-O-hexoside (10) and dimethoxycoumarin-O-hexoside (13). These organs present major peaks attributed to caffeoylquinic acids (9, 15) and various isomers of dicaffeoylquinic acid (28, 34, 37, 38, 40). Less represented hydroxycinnamic acid derivatives include dicaffeoylquinic acid hexoside (25), caffeoylpentoside (17) and three isomers of tricaffeoylquinic acid (60, 65, 68), which were mostly abundant in the leaves. Moreover, this species is strongly characterized by the presence of four ferulic acid derivatives, one with a molecular ion of 693.20 m/z (42) and three isomers showing a molecular ion of 735.21 m/z (58, 62, 64,); the resulting neutral loss of 42.01 Da and the higher retention time strongly suggest that the latter could be the acetylated forms of compound 42. With respect to stems, A. alba leaves presented higher levels of one isomer of a dihydroxybenzoic acid hexoside (4) and various flavonoids glycosides including quercetin-3-O-rutinoside (22), kaempferol-O-hexoside-deoxyhexoside (23), kaempferol-3-O-rutinoside (29), isorhamnetin-O-hexoside-deoxyhexoside (30) and apigenin-O-hexoside-deoxyhexoside (31). Interestingly, according to literature32, we detected high levels in the leaves of A. alba of tuberonic acid hexoside (16), which belong to the class of jasmonate glycosylated derivatives, i.e. phospholipid-derived hormones that regulate plant development and responses to environmental stress.
A. annua (Fig. 2C) was the species presenting the most diversified profile of its secondary metabolome, being characterized by many major compounds belonging to different metabolite classes. In general, the highest diversification was observed within the class of hydroxycinnamic acid derivatives and their esters. In particular, two isomers (12, 20) of o-coumaric acid glucoside (trans- and cis-melilotoside), previously reported in A. annua tea infusions30, were detected at high levels in the leaves together with other hydroxycinnamate esters that include feruloyl moieties, such as two feruloylquinic acid isomers (19, 21), one diferuloylquinic acid isomer (63) and five caffeoylferuloylquinic acid isomers, one characterizing mostly the leaves (50) and the other ones the stems (45, 51, 54, 59). Also, caffeoyl-(9, 15), dicaffeoyl-(33, 34, 37, 38, 40, 61) and tricaffeoyl-(60, 65, 68) quinic acids were well represented in both leaves and stems within this species. Among the coumarins, the stems reported the highest levels of methoxycoumarin-O-hexoside (10). Leaves, on the other hand, were characterized by high amounts of flavonoids, especially methoxylated and often in aglycone form, such as casticin (80), which is described as a marker of A. annua28 and a trihydroxytrimethoxyflavone isomer (77). The latter, together with the methoxylated flavonol mearnsetin-O-hexoside (26), represented the major flavonoid peaks of the A. annua leaf chromatograms. Other characteristic flavonoids included two isomers of a tetrahydroxydimethoxyflavone (66, 74), quercetin-3-O-glucoside (24) and apigenin-7-O-glucoside (39).
Finally, three unidentified metabolites (14, 27, 82) were detected at high levels in A. annua. In particular, compound 27 forms a molecular adduct with formic acid at 389.11 m/z under negative ionization conditions; its fragmentation results in the molecular ion at 343.10 m/z and in the fragment 181.05 m/z, which is indicative of the neutral loss of a hexose (− 162.05 Da). Moreover, the UV–Vis absorbance at 282 and 340 nm together with the fact that it is detected as formic acid adduct, supports the hypothesis that this metabolite could belong to the class of coumarins.
A. verlotiorum (Fig. 2D) and A. vulgaris (Fig. 2E) presented much simpler profiles respect to the other species.
A. verlotiorum was distinct from all the other species for the higher content of two lignans (tracheloside isomers; 46, 53) and three compounds that were putatively annotated as farnesane sesquiterpene acetyl-hexose isomers according to their fragmentation pattern (67, 71, 79). Another peculiarity of this species was the high relative levels of various tricaffeoylhexaric acid isomers (35, 44, 48, 57), including those acylated to isobutyric acid moieties commonly found in other Asteraceae species (69, 70, 72);35 and other tricaffeoyl acid esters (73, 76) that were particularly high in the stems. The leaves and stems were also characterized by many different isomers of dicaffeoyl (18, 28, 33, 34, 37, 38, 40, 41, 55) and tricaffeoyl (60, 65, 68) quinic acid, by dicaffeoylquinic acid hexoside (25) and coumaroylcaffeoylquinic acid isomers (47, 52), while neochlorogenic acid (3) levels were higher in the leaves of this species. Other characteristic metabolites of A. verlotiorum leaves were represented by hexose (1) and pentose (5) esters of a dihydroxybenzoic acid, by quercetin-O-caffeoylhexoside (43) and by four flavone glycosides, which included apigenin-O-hexoside-deoxyhexoside (31), apigenin-7-O-glucoside (39) and two dihydroxymethoxyflavone-O-hexoside isomers (49, 56), the latter best represented in the stems.
A. vulgaris, within the five species, contained lower levels of flavonoids but its stems were characterized by the presence of ginsenoside-like (75) and calenduloside-like (78) saponins. Moreover, leaves were characterized by three dicaffeoylquinic acid isomers (34, 37, 38), which were much more represented in this species respect to other ones, yet at lower amounts. In proportion, also neochlorogenic acid (3), caffeoyl pentoside (17), two dihydroxybenzoic acid hexosides (2, 4) and one sesquiterpene diol monohexoside derivative (79) characterize A. vulgaris aerial organs.
The HCA derivatives, in particular the esters of caffeic acid, were the most characterizing compounds in all the five Artemisia species considered in this work. All these metabolites showed accumulation patterns similar to those already reported in literature for several Artemisia species by using similar extraction methods and identified with different techniques from LC–MS such as NMR and HPLC–DAD7,14,43. The degree and the nature of esterification determines the type of the specific ester isomer that is produced and, for several HCA derivatives, we observed species-specific esterification patterns. This indicates a diversification in the late enzyme of the pathway, among Artemisia spp., such those involving the esterases44. It is interesting to notice how distribution of isomers of different esters of caffeic acid changes also between different organs (stems or leaves) of each species. For instance, various tricaffeoylhexaric acid isomers, a molecule that has been already detected in various Asteraceae45,46, were found at high levels in A. verlotiorum stems, while tricaffeoylquinic acids were located mostly in the leaves. This is probably due to a different spatial distribution in the enzyme involved in the esterification of HCA biosynthesis between the different organs in each species.
As already reported by the literature, flavonoids are another widely represented class of metabolites in the Artemisia genus10,42,43. Ubiquitous presence of different glycosylated and methoxy-glycosylated flavonoids among all the five species investigated has been observed. Interestingly, in A. annua, and to a lesser extent also in A. absinthium and A. alba, aglycone form of different flavonoids, including the methoxylated flavonol casticin, were observed. Within plant cells, most flavonoids are present as O- or C-glycosides47,48, while the aglycones are more typical of extracellular exudates49. The presence of different flavonoid aglycones has been reported in plant exudates of different Artemisia species50,51. For instance, in A. absinthium, A. alba and A. vulgaris, different polymethoxylated flavonoid aglycones were found on the surface of aerial parts, in the extracellular environment, predominantly in leaves and floral buddings, where they probably have protective roles and/or allelopathic functions12,52. The occurrence of these compounds is probably related to the presence on the leaf surface of epidermal trichomes, which can synthetize and store large quantities of specialized metabolites53. Trichomes are epidermis appendages and can be divided into glandular trichomes (GTs) and non-glandular (NGTs) according to their morphology54. In particular, glandular trichomes can synthesize, store, and secrete large amounts of exudates, including alkaloids, polysaccharides, terpenoids, polyphenols, organic acids, and defensive proteins. In turn, these exudates can entrap or poison herbivores and prevent pathogen infection55,56. Usually Asteraceae, harbors mainly GTs, where high-value secondary metabolites, including artemisinin in A. annua, are produced and then stored, ready to be used in plant defensive mechanism against both biotic and abiotic stress54,57. Presence of glandular trichomes, has been reported for all the species investigated in this work, and can explain the observation of the above mentioned flavonoid aglycones in our samples18,58.
Considering the three different growing seasons, the strongest differences were observed for A. annua and A. verlotiorum. In A. annua, the relative levels of many flavonoids and hydroxycinnamic acid derivatives during 2019 was lower than 2020 and 2021. In A. verlotiorum, some flavonoids and various hydroxycinnamic acid derivatives showed higher relative level in 2021 compared with 2019 and 2020. This variation could be expected for herbaceous annual species14, such as A. annua, if we consider that the sampling of potentially distinct individuals over 3 years could have increased the genotypic variability of the samples. It is not excluded that some Artemisia species are more sensitive to environmental conditions than others and modulate the levels of single or groups of metabolites in response to different stimuli. However, the type of experimental design that we adopted in this work does not allow us to precisely dissect the effects of specific climate or geographic conditions and, thus, is not suitable to investigate such complex environment-metabolome interactions.
Antioxidant assays of Artemisia spp. methanolic extracts
In this work we performed in vitro antioxidant assays as low cost and easy to use high-throughput screening systems for the identification of potential sources of antioxidants59,60. These should then be followed by confirmatory in vivo biological tests with simulated digestion samples61 to assess the antioxidant activity in a more physiological context. It is commonly accepted that antioxidant activity must not be tested on the basis of a single method62 given the involvement of different antioxidant mechanisms by the molecules present in a phytocomplex. Thus, we used FRAP and DPPH to assess the reducing capacity and radical scavenging activity, respectively, of leaf and stem methanolic extracts of the five Artemisia species.
In general, extracts from leaves showed higher antioxidant capacity compared with extracts from stems in both assays (Fig. 4).
Antioxidant activity of extracts from Artemisia spp. leaves and stems, sampled in three independent growing seasons, and determined by FRAP (A, B) and DPPH (C, D) assays, and expressed as Trolox Equivalent Antioxidant Capacity (TEAC), in millimoles of Trolox Equivalents/kg of tissue (leaves or stem), fr. wt. Values are expressed as mean +/− standard deviation (n = 9). Significant differences were calculated with one-way ANOVA.
Within the five species, A. absinthium showed the lowest antioxidant activity (up to 30.0 and 24.8 mmolTE/Kg fr. wt in FRAP and DPPH), while A. verlotiorum showed the highest antioxidant power for both FRAP (121.2 mmolTE/Kg fr. wt) and DPPH (up 88.7 mmolTE/Kg fr.wt), about four times higher than A. absinthium. In between these two species, A. annua, A. alba and A. vulgaris displayed a medium to high antioxidant activity (see Supplementary Table 1).
Many studies report the antioxidant activity of different Artemisia spp. extracts assayed with a broad panel of methods but a comparison with the results presented in this work is challenged by non-homogeneous expression of data (e.g. TEAC, IC50, percentage of radical scavenging, etc.) or the use of reference compounds other than Trolox. A few recent studies report the antioxidant activity of methanolic extracts of various Artemisia species in comparison to Trolox19,28,63 (Supplementary Table 2). Our results are in line with the trend observed by Trifan and colleagues for FRAP assay, in which A. absinthium displayed the lowest antioxidant activity. On the other hand, we did not observe higher antioxidant activity for A. vulgaris, as reported by the authors. In general, the TEAC values reported in all these studies for the five Artemisia species are five to ten times higher than our results, but this is justified by the fact that dried instead of fresh plant material was used to produce the extracts, thus resulting in higher concentrations of antioxidant compounds.
In Fig. 5 the antioxidant activity in each of the 3 years of sampling is shown (see also Supplementary Table 1). In some cases, a clear impact of the specific growing season on antioxidant activity was observed. For example, A. verlotiorum extracts showed higher antioxidant activities in 2021 than 2019 and 2020 in both FRAP (Fig. 5G) and DPPH (Fig. 5H), while A. annua and A. vulgaris showed lower antioxidant activities in 2019 compared with 2020 and 2021 (Fig. 5E, F, I, L). On the other hand, the antioxidant activity of the leaves of A. absinthium (Fig. 5A, B) and A. alba (Fig. 5C, D), did not vary significantly throughout the 3 years. According to these data, the antioxidant activity of species like A. absinthium and A. alba seems to be less influenced by the growing season as it occurs in the case of A. annua, A. vulgaris and A. verlotiorum.
Antioxidant activity of extracts from Artemisia spp. leaves and stems, sampled in three independent growing seasons, and determined by FRAP (A, C, G, I) and DPPH (B, D, F, H, J) assays, and expressed as Trolox Equivalent Antioxidant Capacity (TEAC), in millimoles of Trolox Equivalents/kg of tissue (leaves or stem), fresh weight. Values are expressed as mean +/− standard deviation (n = 9). Aab, absinthium; Aal, alba; Aan, annua; Ave, verlotiorum; Avu, vulgaris. Significant differences calculated with two-way ANOVA.
Correlation analysis of antioxidant data and metabolic profiles of Artemisia spp.
In order to obtain information on which metabolites may be responsible for antioxidant activity of Artemisia spp. methanolic extracts, a statistical tool of multivariate analysis (OPLS) was used to find linear relations between the metabolite levels (whose m/z features were assigned as X variables) and the antioxidant capacity (whose mmolTE/Kg were assigned as Y variables). The score scatter plots of Fig. 6 show a good, yet not too strong, linear correlation between the metabolite levels (t, x axis) and the antioxidant activity (u, y axis), for both FRAP and DPPH (0.86 < R2 < 0.90), thus recalling the need for an independent OPLS analysis in each different species; this is expected, since different set of metabolites could be responsible for the overall antioxidant activity of each species. In this analysis, samples that displayed the highest mmol TE/Kg of fresh plant material clustered on the top right corner of the graph and those with the lower values in the left-down corner.
The same analysis was applied to each of the individual species. The OPLS individual models for leaves and stem extracts of each of the species are shown in Fig. 7. The loadings of these OPLS analyses can be used to evaluate the contribution of each m/z feature, i.e. of each detected metabolite, to the observed antioxidant activity (Tables 3, 4).
Scatter plot of OPLS analysis that correlates antioxidant activity (u) (FRAP on the left and DPPH on the right) with metabolic composition (t). Samples are colored according to the organs. (A) A. absinthium (Aab); (B) A. alba (Aal); (C) A. annua (Aan); (D) A. verlotiorum (Ave); (E) A. vulgaris (Avu).
Consistently to what observed in the previous paragraphs, the antioxidant activity of leaf extracts is in general higher than those of stem extracts (Fig. 7). This comparative analysis between the species, showed that the higher antioxidant activity of A. verlotiorum, described in the previous paragraph, may be mainly due to caffeic and ferulic acid derivatives and flavonoids; also, various unidentified metabolite showed high correlation with antioxidant activity (data not shown). In A. vulgaris the antioxidant activity correlated with caffeic acid derivatives, in A. annua, with coumaric, caffeic and ferulic acid derivatives. In A. alba, which showed the lowest linear correlation between metabolome composition and antioxidant activity (Fig. 7), also flavonoids were found to strongly correlate with antioxidant activity in FRAP assay, while the scavenging activity measured by DPPH was mostly correlated with dicaffeoylquinic acid isomers. Interestingly, coumarins accumulated at high levels in A. alba but did not strongly correlate with the antioxidant activity. Finally, in A. absinthium the antioxidant activity strongly correlated with caffeoylquinic acids, absinthin and flavonoids.
The hydroxycinnamates esterified with quinic acid, in particular some isomers of dicaffeoylquinic acid, were found to be metabolites with the strongest correlation with antioxidant activity in all species. This class of molecules has been extensively studied in the past years for their potential use in medicine. Caffeoylquinic acid derivatives are natural compounds isolated from a variety of traditional medicinal plants and possess a wide range of pharmacological properties, including antioxidant, hepatoprotective, antibacterial, antihistaminic and other biological effects64. Currently, in literature, caffeoyl and dicaffeoylquinic acids have been widely tested through in vitro and in vivo assays to evaluate their bioactive properties. Two caffeoylquinic acids extracted from Aronia melanocarpa berries, i.e. 3-caffeoylquinic acid and 4-caffeoylquinic acid, were identified as inhibitor of the dipeptidyl peptidase IV, an enzyme involved in the development of type 2 diabetes mellitus65. The protective effect of chlorogenic acid against neurotoxic effect of arsenic poisoning was demonstrated in mice model66. Potential benefits with therapeutic applications were reported also for dicaffeoylquinic acids. For example, Kim and collaborators demonstrated the neuroprotective effect of 3,5-dicaffeoylquinic acid and 3,4-dicaffeoylquinic acid from Dipsacus asper on hydrogen peroxide-induced cell death in SH-SY5Y human cells67. In another study it is reported that 1,5-dicaffeoylquinic acid (cynarin) downregulates the expression of inducible nitric oxide synthase, expressed under conditions of inflammation, sepsis, or oxidative stress, in human coronary smooth muscle cells68. In addition, the dicaffeoyl quinic acid cynarin affects the survival, growth, and stress response of normal, immortalized, and cancerous human cells69. Protective effects of cynarin against hepatoxicity effects of cyclophosphamide, an important anticancer drug which belongs to the class of alkylating agents, as well as its antihypertensive and vasodilator effects have been observed in in vivo studies70,71. Artemisia spp., which were able to accumulate various isomers of caffeoyl- and dicaffeoylquinic acid, could thus represent a valuable source of these potentially bioactive compounds. A further characterization to reveal the precise identity of the various isomers accumulated by the five species is thus required.
Looking for a new artemisinin source
The sesquiterpene lactone artemisinin and its semi-synthetic derivatives are very important from a pharmaceutical perspective for their anti-malarial properties. Isolated from A. annua plants, artemisinin earned in short time the status of most potent antimalarial drug and recently new evidence of many other bioactivities (e.g. anticancer, anti-inflammatory and antiviral) have emerged72. For this reason, a great interest arose in the search for artemisinin-rich A. annua ecotypes and towards the manipulation of its biosynthetic pathway through different biotechnological tools21. Moreover, since antimalarial activity was reported for different Artemisia species15, many studies have been conducted to find alternative natural sources for artemisinin within the Artemisia genus. Despite artemisinin was demonstrated to occur in different amounts in A. dubia73, A. scoparia74, A. cina75, A. vachanica and A. dracunculus76, A. verlotiorum and A. vulgaris77, the major source of this metabolite still remains A. annua78.
In this work we explored the capacity of the Artemisia plants collected in the province of Verona to produce the antimalaria lead drug artemisinin and related compounds from its biosynthesis pathway. We therefore performed an LC–MS analysis in positive ionization mode, which is more suitable for the ionization of sesquiterpenoid molecules, and we searched for the final products of the pathway (artemisinin and arteannuin B) and their immediate precursors (dihydroartemisinic acid and artemisinic acid, respectively). Their identification was made through the comparison of m/z values, fragmentation patterns and retention times with those of the respective reference standards (Table 5). The relative comparison of their levels within the leaves of the five Artemisia species is reported in Fig. 8.
A. annua is the only species reporting detectable levels of artemisinin. Interestingly, the precursor of artemisinin, dihydroartemisinic acid, is present not only in A. annua but also in A. alba and A. verlotiorum. Arteannuin B, the final metabolite of a parallel pathway that originates from artemisinic aldehyde, was detected at high levels in A. annua and at considerably lower levels in A. absinthium and A. alba but was absent in A. verlotiorum. The precursor of both arteannuin B and artemisinin, artemisinic acid, is present in A. annua, as expected, but we did not detect it in A. absinthium nor A. alba, despite the fact that arteannuin B has been detected in both species. A. vulgaris does not produce any of the metabolites from the selected pathway.
According to the literature, the presence of artemisinin has been recently reported in A. verlotiorum77, A. absinthium15 and A. vulgaris77. While peak in artemisinin content in A. annua and A. vulgaris corresponds to the budding stage, in A. absinthium maximum accumulation is reached during the flowering stage78,79,80. This could explain the absence of artemisinin, or its eventual presence below detectable levels, in all the plants used in our work which were sampled during the vegetative growth. On the other hand, we reported for the first time the presence of artemisinin intermediates in A. alba, thus furtherly increasing the number of sesquiterpene compounds (e.g. germacrane and eudesmane) that were previously reported in this species20.
Although we did not detect traces of artemisinin, the presence of the precursor, dihydroartemisinic acid, and side product of the pathway, arteannuin B, indicates that genes of artemisinin biosynthetic pathway are all expressed in these plants, since the last step of artemisinin biosynthesis is a non-enzymatic photooxidative process81. The hypothesis that the artemisinin biosynthetic pathway may be an ancestral characteristic shared among many plants within genus Artemisia15 is supported by recent reports that confirmed the expression of structural genes in eight Artemisia species, including A. absinthium and A. vulgaris78. Their expression levels varied specifically depending on the organ collected and the developmental phase considered, with their expression ratio and turnover being crucial to address the flux of intermediates through the two branches of the pathway, leading in turn to artemisinin or arteannuin B accumulation. Nonetheless, the complexity of the physiological context linked to artemisinin accumulation recall the need to consider other factors to explain the large variability observed in the production of artemisinin-related metabolites78. For instance, the expression of transcription factors affecting structural genes and glandular trichome formation, which represent the site for artemisinin production and storage, have been recently investigated and considered as a target for metabolic engineering approaches to increase artemisinin levels81. In addition, the expression of other sesquiterpene synthases diverting the carbon resources of farnesyl-diphosphate into other competing pathways has to be considered.
Extending these transcriptomics analyses not only to A. annua but also to other species within the genus82 will provide useful molecular information to decipher, together with metabolomics data, the different biosynthetic capabilities of Artemisia spp. in artemisinin-related sesquiterpenes production and accumulation.
Experimental section
Plant material
The five selected Artemisia species were sampled independently throughout three growing seasons in June of the years 2019, 2020, 2021 from three hills and mountain areas in the province of Verona (Supplementary Table 3). Following identification, the plants were given the barcode number and a voucher specimen of each species is available at the “Museo di Storia Naturale” of Verona. All sampling procedures were conducted in accordance to the guidelines.
In each sampling site, plants were collected from three distinct spots (i.e. three different plant populations representing three biological replicates), far enough to avoid the sampling of plant populations deriving from the same genetic source. For each replicate, leaves and stems were collected from at least 5 individuals and pooled together according to the organ. The samples were immediately frozen in dry ice and then stored at − 80 °C. The frozen plant material was homogenized in liquid nitrogen using an IKA A11 basic mill (IKA, Germany).
Chemicals and reagents
Reference standard of artemisinin was purchased from Sigma-Aldrich (St. Louis, USA). Reference standards of arteannuin B and artemisinic acid were purchased from Biosynth® Carbosynth (Bratislava, Slovakia). Reference standard of dihydroartemisinic acid was purchased from Toronto Reasearch Chemical (Toronto, Canada). Methanol, acetonitrile and water (all LC–MS grade) were purchased from Honeywell (Charlotte, USA). Formic acid (LC–MS grade) was purchased from Biosolve Chimie (Dieuze, France). Trolox and DPPH were purchased, respectively, from Sigma-Aldrich and Thermo Fisher Scientific.
Metabolites extraction
100 mg of frozen powder were extracted in 1.5 ml methanol, mixed vigorously for 30 s, sonicated at 40 kHz in an ultrasonic bath Sonica® Ultrasonic Cleaner (SOLTEC, Milan, Italy) at 4 °C for 15 min and centrifuged (16,000g, 15 min, 4 °C). The supernatants were opportunely diluted in 100% methanol. Just before the analysis, the samples were diluted 1:2 (V:V) with ultrapure water (Honeywell, USA). The final mixtures were passed through Minisart RC4 filters (0.2 μm pores) (Sartorious, Göttingen, Germany) and 1 μl was injected into the UPLC device.
UPLC–ESI–MS analysis
An Acquity I Class UPLC system (Waters, Milford, USA) with a BEH C18 column (Waters), coupled online with a PDA (photo-diode array) and to a Xevo G2-XS qTOF mass spectrometer (Waters), equipped with an electrospray ionization (ESI) source were used. The extracts were injected through a cooled autosampler (8 °C) and a flow rate of 0.350 ml/min was used. The mobile phases were 0.1% formic acid in water (solvent A) and acetonitrile (solvent B), and the elution gradient was as follows: 0–1 min, 1% B; 1–10 min, 1–40% B ; 10–13.50 min, 40–70% B; 13.50–15.00 min, 70–90% B; 15.00–16.50 min, 90–100% B; 16.50–20 min 100% B; 20–20.1, 100–1% B; 20.1–25 min, 1% B (initial conditions).
The sample analysis sequence was randomized. A quality control (QC) prepared by mixing equal part of all the extracts was analyzed along the whole experiment every ten sample analysis. The ion source parameters were the following: capillary voltage 0.8 kV, sampling cone voltage 40 V, source offset voltage 80 V, source temperature 120 °C, desolvation temperature 500 °C, cone gas flow rate 50 l/h and desolvation gas flow rate 1000 l/h. Nitrogen gas was used for the nebulizer and in desolvation whereas argon was used to generate collision-induced dissociation. MS data were acquired in continuum in both negative and positive ionization mode within the range 50–2000 m/z using a fixed collision energy of 35 V. Data were acquired through the Mass Lynx v4.2 software (Waters).
Processing of LC–MS data and metabolites identification
The chromatograms were manually inspected through Mass Lynx software. Metabolites were identified by relying on m/z value of the monoisotopic molecular ion, retention time and MS/MS fragmentation pattern by comparison with an in-house library of authentic standard. When no standard compounds were available, the identification was tentatively assigned comparing m/z, isotopic ratio, fragmentation pattern and UV/vis absorbance spectra with those reported in scientific literature and public databases (Chemspider, Human Metabolome Database, Lotus Natural Products, MassBank, MoNA, Pubchem, etc.). In particular, for the characterization of caffeoyl ester derivatives and various glycosides the following neutral losses (Da) were considered: 132.042 (pentose), 146.058 (deoxyhexose), 162.032 (caffeic acid moiety), 162.053 (hexose),
The chromatograms acquired in negative ionization mode were processed with Progenesis QI software (Waters) to obtain the Feature Quantification Matrix (FQM; Supplementary File 1).
Antioxidant assays
The same methanolic extracts used for UPLC-ESI–MS analysis were used for determination of antioxidant activity in vitro by FRAP and DPPH assays in transparent 96-well microplates.
A FRAP solution was prepared mixing in a ratio of 10:1:1 (V:V:V) the following reagents: FRAP buffer (3.1 g/l sodium acetate trihydrate, 16 ml/l acetic acid pH 3.6), 10 mM TPTZ (2,4,6-tri(2-pyridyl)-1,2,5-triazine) in HCl 40 Mm, FeCl3 * 6H2O 20 Mm , The test was carried out mixing 200 µl of the FRAP solution to 20 µl of the sample, or solutions of Trolox at different concentrations or methanol (blank). Methanolic extracts of samples were diluted 1:20 for leaves and in a range from 1:3 to 1:10 for stems. Each sample was tested in three technical replicates. The microplate was incubated at 37 °C in the dark for 15 min and then kept cooling at room temperature for 4 min. The absorbance was measured at 593 nm using the Infinite 200 PRO plate reader (Tecan, Männedorf, Switzerland).
1 mM DPPH stock solution was freshly prepared in methanol at least 2 h before the assay. 100 µM of working solution was prepared diluting 1:10 (V:V) in 70% methanol the DPPH solution. 200 µl of the DPPH solution were added to 20 µl of the sample, i.e. diluted plant extracts or solutions of Trolox at different concentrations or methanol (blank). Methanolic extracts were diluted in a range from 1:10 to 1:20 and from 1:3 to 1:10 for leaves and stems, respectively. Each sample was tested in three technical replicates. The microplate was incubated at 25 °C in the dark for 30 min and then the absorbance was measured at 517 nm using the Infinite 200 PRO plate reader (Tecan).
The compound Trolox, a water-soluble Vitamin E analogue, was used as reference antioxidant in order to express the antioxidant power of the plant extracts, expressed as Trolox Equivalent Antioxidant Capacity (TEAC), whose unit is mmol of Trolox Equivalent for Kg (mmol/kg). 20 µl of Trolox solutions with concentrations spanning from 500 to 5 µM was added to 200 µl of FRAP or DPPH solution to generate a Trolox calibration curve in each assay.
Statistical analysis
The FQM and antioxidant (TEAC values) data were analyzed with SIMCA-P software (Umetrics, Sweden) for multivariate statistical analysis in order to look for relationships among the in-vitro antioxidant activity of the plant extracts and their metabolite composition. The m/z features (i.e. the metabolites) of the dataset were assigned as X variables (Pareto scaling) and the antioxidant activity as Y variables (UV scaling). Orthogonal Partial Least Square (OPLS) analysis was used. Metabolites putatively responsible for the antioxidant activity were identified by inspection of the column loading plot; only metabolites showing a pq(corr) value > 0.8 (arbitrary threshold) were considered correlated with antioxidant activity. All statistical calculations were performed using the GraphPad Prism version 8.0 software (GraphPad Software, San Diego, California USA). The means values ± SD (n = 3) are reported in the figures. Statistical analyses were conducted using One or Two-way Anova followed by Tukey’s Test.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Oyemitan, I. A. African Medicinal Spices of Genus Piper 581–597 (Elsevier, 2017).
Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 19, 1199–1209 (2020).
Garnatje, T., Peñuelas, J. & Vallès, J. Ethnobotany, phylogeny, and ‘omics’ for human health and food security. Trends Plant Sci. 22, 187–191 (2017).
Su, X.-Z. & Miller, L. H. The Discovery of Artemisinin and the Nobel Prize in Physiology or Medicine (Springer, Berlin, 2015).
Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220 (2011).
Rønsted, N. et al. Can phylogeny predict chemical diversity and potential medicinal activity of plants? A case study of Amaryllidaceae. BMC Evol. Biol. 12, 1–12 (2012).
Bora, K. S. & Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 49, 101–109. https://doi.org/10.3109/13880209.2010.497815 (2011).
Koul, B. & Khatri T. The Artemisia genus: Panacea to several maladies. Bioactive Nat. Prod. Drug Discov. 3–95 (2020).
Martínez, M. J. A., Del Olmo, L. M. B., Ticona, L. A. & Benito, P. B. The Artemisia L. genus: A review of bioactive sesquiterpene lactones. Stud. Nat. Prod. Chem. 37, 43–65 (2012).
Bisht, D., Kumar, D., Kumar, D., Dua, K. & Chellappan, D. K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharmacal Res. 44, 439–474 (2021).
Pandey, A. K. & Singh, P. The genus Artemisia: A 2012–2017 literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 4, 68 (2017).
Trendafilova, A., Moujir, L. M., Sousa, P. M. & Seca, A. M. Research advances on health effects of edible Artemisia species and some sesquiterpene lactones constituents. Foods 10, 65 (2020).
Chen, L., Qiu, J. & Tarolli, P. Water resources management of grasslands under climate change in the historic rural landscape of Lessinia Regional Park (Veneto, Italy). In EGU General Assembly Conference Abstracts, 2022, p. EGU22–7963.
Nigam, M. et al. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 14, 1934578X19850354 (2019).
Pellicer, J. et al. A phylogenetic road map to antimalarial Artemisia species. J. Ethnopharmacol. 225, 1–9. https://doi.org/10.1016/j.jep.2018.06.030 (2018).
Bora, K. S. & Sharma, A. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm. Biol. 49, 1216–1223. https://doi.org/10.3109/13880209.2011.578142 (2011).
Obistioiu, D. et al. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 8, 6. https://doi.org/10.1186/1752-153X-8-6 (2014).
Peron, G. et al. Phytochemical investigations on Artemisia alba Turra growing in the North-East of Italy. Nat. Prod. Res. 31, 1861–1868. https://doi.org/10.1080/14786419.2016.1263845 (2017).
Ickovski, J. D., Mitić, M., Stojković, M. & Stojanović, G. Comparative analysis of HPLC profiles and antioxidant activity of Artemisia alba Turra from two habitats in Serbia. Chemia Naissensis 3, 89–95 (2020).
Todorova, M. et al. Highly oxygenated sesquiterpenes in Artemisia alba Turra. Phytochemistry 110, 140–149. https://doi.org/10.1016/j.phytochem.2014.12.008 (2015).
Kayani, W. K., Kiani, B. H., Dilshad, E. & Mirza, B. Biotechnological approaches for artemisinin production in Artemisia. World J. Microbiol. Biotechnol. 34, 1–14 (2018).
Crespo-Ortiz, M. P. & Wei, M. Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. BioMed Res. Int. 2012 (2012).
Bittencourt De Souza, L. F. et al. Genotoxic potential of aqueous extracts of Artemisia verlotorum on the cell cycle of Allium cepa. Int. J. Environ. Stud. 67, 871–877. https://doi.org/10.1080/00207233.2010.520457 (2010).
Mootoosamy, A. & Fawzi, M. M. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J. Ethnopharmacol. 151, 413–444. https://doi.org/10.1016/j.jep.2013.10.069 (2014).
Ekiert, H. et al. Significance of Artemisia Vulgaris L. (common mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies. Molecules 25, 4415. https://doi.org/10.3390/molecules25194415 (2020).
Natividad, G. M. et al. Actions of Artemisia vulgaris extracts and isolated sesquiterpene lactones against receptors mediating contraction of guinea pig ileum and trachea. J. Ethnopharmacol. 137, 808–816. https://doi.org/10.1016/j.jep.2011.06.042 (2011).
Lee, S.-J. et al. Estrogenic flavonoids from Artemisia vulgaris L. J. Agric. Food Chem. 46, 3325–3329 (1998).
Trifan, A. et al. Unveiling the phytochemical profile and biological potential of five Artemisia species. Antioxidants 11, 1017. https://doi.org/10.3390/antiox11051017 (2022).
Mamatova, A. S. et al. Phytochemical composition of wormwood (Artemisia gmelinii) extracts in respect of their antimicrobial activity. BMC Complement. Altern. Med. 19, 288. https://doi.org/10.1186/s12906-019-2719-x (2019).
Mouton, J. & van der Kooy, F. Identification of cis-and trans-melilotoside within an Artemisia annua tea infusion. Eur. J. Med. Plants 4, 52–63 (2014).
El-Wassimy, M. T. M., Ahmed, M. M., Younes, S. H. H. & Hegazy, M.-E.F. Antimicrobial and Antiproliferative activity of Tomenin compound isolated from Seriphidium Herba-Alba. Sohag J. Sci. 3, 1–8. https://doi.org/10.21608/sjsci.2018.233272 (2018).
Melguizo-Melguizo, D., Diaz-de-Cerio, E., Quirantes-Piné, R., Švarc-Gajić, J. & Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 10, 192–200. https://doi.org/10.1016/j.jff.2014.05.019 (2014).
Han, J. et al. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 47, 516–525. https://doi.org/10.1016/j.jpba.2008.02.013 (2008).
Ghareeb, M. A. et al. HPLC-ESI-MS/MS profiling of polyphenolics of a leaf extract from Alpinia zerumbet (Zingiberaceae) and its anti-inflammatory, anti-nociceptive, and antipyretic activities in vivo. Molecules 23, 3238. https://doi.org/10.3390/molecules23123238 (2018).
Kłeczek, N., Malarz, J., Gierlikowska, B., Kiss, A. K. & Stojakowska, A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and anti-inflammatory activity of 7, 10-diisobutyryloxy-8, 9-epoxythymyl isobutyrate. Molecules 25, 4913 (2020).
Zhang, C. et al. Integration of data-dependent acquisition (DDA) and data-independent high-definition MSE (HDMSE) for the comprehensive profiling and characterization of multicomponents from Panax japonicus by UHPLC/IM-QTOF-MS. Molecules 24, 2708. https://doi.org/10.3390/molecules24152708 (2019).
Boukhalkhal, S., Gourine, N., Pinto, D. C. G. A., Silva, A. M. S. & Yousfi, M. UHPLC-DAD-ESI-MSn profiling variability of the phenolic constituents of Artemisia campestris L. populations growing in Algeria. Biocatal. Agric. Biotechnol. 23, 101483. https://doi.org/10.1016/j.bcab.2019.101483 (2020).
Grati, W. et al. HESI-MS/MS analysis of phenolic compounds from Calendula aegyptiaca fruits extracts and evaluation of their antioxidant activities. Molecules 27, 2314. https://doi.org/10.3390/molecules27072314 (2022).
Su, L. -H., Ma, W. -J., Ma, Y. –B., Li, T. –Z., Geng, C. –A., Dong, W., et al. Artemiprincepsolides A–F, novel germacrane‐guaiane and eudesmane‐guaiane sesquiterpenoid dimers from Artemisia princeps and their antihepatoma activity. Chin. J. Chem. n.d.
Dong, W, Ma, W. -J., Ma, Y. –B., Li, F. –J., Li, T. –Z., Wang, Y. –C., et al. Guaiane‐type sesquiterpenoid dimers from Artemisia zhongdianensis and antihepatoma carcinoma activity via the p38MAPK pathway. Chin. J. Chem. (2023).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 3, 211–221. https://doi.org/10.1007/s11306-007-0082-2 (2007).
Aberham, A., Cicek, S. S., Schneider, P. & Stuppner, H. Analysis of sesquiterpene lactones, lignans, and flavonoids in wormwood (Artemisia absinthium L.) using high-performance liquid chromatography (HPLC)-mass spectrometry, reversed phase HPLC, and HPLC-solid phase extraction-nuclear magnetic resonance. J. Agric. Food. Chem. 58, 10817–10823. https://doi.org/10.1021/jf1022059 (2010).
Carvalho, I. S., Cavaco, T. & Brodelius, M. Phenolic composition and antioxidant capacity of six artemisia species. Ind. Crops Prod. 33, 382–388. https://doi.org/10.1016/j.indcrop.2010.11.005 (2011).
El-Seedi, H. R. et al. Hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in Islamic medicine. Stud. Nat. Prod. Chem. 55, 269–292 (2018).
Kłeczek, N., Michalak, B., Malarz, J., Kiss, A. K. & Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. revisited: Newly identified constituents from aerial parts of the plant and their possible contribution to the biological activity of the plant. Molecules 24, 1614. https://doi.org/10.3390/molecules24081614 (2019).
Kramberger, K. et al. HPLC-DAD-ESI-QTOF-MS determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species. Metabolites 10, 403. https://doi.org/10.3390/metabo10100403 (2020).
Slámová, K., Kapešová, J. & Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 19, 2126. https://doi.org/10.3390/ijms19072126 (2018).
Viskupičová, J., Ondrejovič, M. & Šturdík, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 47, 151–162 (2008).
Wollenweber, E., Dörr, M. & Christ, M. Flavonoid aglycones from the leaf and stem exudates of some Geraniaceae species. Nat. Prod. Commun. 6, 1934578X1100600105. https://doi.org/10.1177/1934578X1100600105 (2011).
Olennikov, D. N. et al. Caffeoylquinic acids and flavonoids of fringed sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS profile, HPLC-DAD quantification, in vitro digestion stability, and antioxidant capacity. Antioxidants 8, 307. https://doi.org/10.3390/antiox8080307 (2019).
Valant-Vetschera, K. M., Fischer, R. & Wollenweber, E. Exudate flavonoids in species of Artemisia (Asteraceae—Anthemideae): New results and chemosystematic interpretation. Biochem. Syst. Ecol. 31, 487–498. https://doi.org/10.1016/S0305-1978(02)00178-3 (2003).
Nikolova, M. & Velickovic, D. Phenological variations in the surface flavonoids of Artemisia vulgaris L. and Artemisia absinthium L. n.d.
Tissier, A. Glandular trichomes: What comes after expressed sequence tags?. Plant J. 70, 51–68. https://doi.org/10.1111/j.1365-313X.2012.04913.x (2012).
Xiao, L., Tan, H. & Zhang, L. Artemisia annua glandular secretory trichomes: The biofactory of antimalarial agent artemisinin. Sci. Bull. 61, 26–36 (2016).
Huchelmann, A., Boutry, M. & Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological interest. Plant Physiol. 175, 6–22. https://doi.org/10.1104/pp.17.00727 (2017).
Tissier, A. Harnessing plant trichome biochemistry for the production of useful compounds. Mol. Pharming Appl. Chall. Emerg. Areas 353–82 (2018).
Liu, Y., Jing, S.-X., Luo, S.-H. & Li, S.-H. Non-volatile natural products in plant glandular trichomes: Chemistry, biological activities and biosynthesis. Nat Prod Rep 36, 626–665. https://doi.org/10.1039/C8NP00077H (2019).
Minarchenko, V. et al. Diagnostic micromorphological features of leaf surface of some Artemisia species (Asteraceae). Hacquetia 22, 131–141 (2023).
Pisoschi, A. M. & Negulescu, G. P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. https://doi.org/10.4172/2161-1009.1000106 (2012).
Tan, J. B. L. & Lim, Y. Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 172, 814–822. https://doi.org/10.1016/j.foodchem.2014.09.141 (2015).
Granato, D. et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods?. Food Chem. 264, 471–475. https://doi.org/10.1016/j.foodchem.2018.04.012 (2018).
Munteanu, I. G. & Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 22, 3380. https://doi.org/10.3390/ijms22073380 (2021).
Suroowan, S. et al. Phytochemical characterization, anti-oxidant, anti-enzymatic and cytotoxic effects of Artemisia verlotiorum Lamotte extracts: A new source of bioactive agents. Molecules 27, 5886 (2022).
Alcázar Magaña, A., Kamimura, N., Soumyanath, A., Stevens, J. F. & Maier, C. S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 107, 1299–1319. https://doi.org/10.1111/tpj.15390 (2021).
Imai, M. et al. Caffeoylquinic acids from aronia juice inhibit both dipeptidyl peptidase IV and α-glucosidase activities. LWT 129, 109544. https://doi.org/10.1016/j.lwt.2020.109544 (2020).
Metwally, D. M. et al. Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J. Funct. Foods 75, 104202. https://doi.org/10.1016/j.jff.2020.104202 (2020).
Kim, S.-S., Park, R.-Y., Jeon, H.-J., Kwon, Y.-S. & Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 19, 243–245. https://doi.org/10.1002/ptr.1652 (2005).
Xia, N. et al. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules 19, 3654–3668. https://doi.org/10.3390/molecules19033654 (2014).
Gezer, C., Yücecan, S. & Rattan, S. Artichoke compound cynarin differentially affects the survival, growth, and stress response of normal, immortalized, and cancerous human cells. Turk. J. Biol. 39, 299–305. https://doi.org/10.3906/biy-1407-67 (2015).
Hakkou, Z. et al. Antihypertensive and vasodilator effects of methanolic extract of Inula viscosa: Biological evaluation and POM analysis of cynarin, chlorogenic acid as potential hypertensive. Biomed. Pharmacother. 93, 62–69. https://doi.org/10.1016/j.biopha.2017.06.015 (2017).
Tong, J. et al. The protective effects of Cichorium glandulosum seed and cynarin against cyclophosphamide and its metabolite acrolein-induced hepatotoxicity in vivo and in vitro. Food Funct. 8, 209–219. https://doi.org/10.1039/c6fo01531j (2017).
Zyad, A., Tilaoui, M., Jaafari, A., Oukerrou, M. A. & Mouse, H. A. More insights into the pharmacological effects of artemisinin. Phytother. Res. 32, 216–229. https://doi.org/10.1002/ptr.5958 (2018).
Kiani, B. H., Safdar, N., Mannan, A. & Mirza, B. Comparative Artemisinin analysis in Artemisia dubia transformed with two different Agrobacteria harbouring rol ABC genes. Plant Omics 5, 386–391 (2012).
Singh, A. & Sarin, R. Artemisia scoparia: A new source of artemisinin. Bangladesh J. Pharmacol. 5, 17–20 (2010).
Ermayanti, T. M., Bintang, M. & Mariska, I. Production of antileukemic agent in untrasformed and transformed root culture of Artemisia cina. Ann. Bogorienses 8 (2001)
Numonov, S. et al. Assessment of artemisinin contents in selected Artemisia species from Tajikistan (Central Asia). Medicines 6, 23 (2019).
Singh, P. et al. Determination of bioactive compounds of Artemisia Spp. plant extracts by LC–MS/MS technique and their in-vitro anti-adipogenic activity screening. J. Pharm. Biomed. Anal. 193, 113707. https://doi.org/10.1016/j.jpba.2020.113707 (2021).
Ranjbar, M., Naghavi, M. R., Alizadeh, H. & Soltanloo, H. Expression of artemisinin biosynthesis genes in eight Artemisia species at three developmental stages. Ind. Crops Prod. 76, 836–843 (2015).
Mannan, A., Ahmed, I., Arshad, W., Hussain, I. & Mirza, B. Effects of vegetative and flowering stages on the biosynthesis of artemisinin in Artemisia species. Arch. Pharmacal Res. 34, 1657–1661 (2011).
Towler, M. J. & Weathers, P. J. Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Ind. Crops Prod. 67, 185–191. https://doi.org/10.1016/j.indcrop.2015.01.007 (2015).
Zhao, L. et al. From plant to yeast—Advances in biosynthesis of artemisinin. Molecules 27, 6888. https://doi.org/10.3390/molecules27206888 (2022).
Salehi, M., Karimzadeh, G., Naghavi, M. R., Naghdi Badi, H. & Rashidi, M. S. Expression of key genes affecting artemisinin content in five Artemisia species. Sci. Rep. 8, 12659. https://doi.org/10.1038/s41598-018-31079-0 (2018).
Acknowledgements
This work was funded by European UNion—NextGenerationEU in the framework of National Biodiversity Future Center and by the University of Verona with the call “Joint Project 2019”.
Author information
Authors and Affiliations
Contributions
S.N., F.P., S.A., C.R., L.A. performed the sampling. S.N., R.N. performed the bioactivity tests, M.C. and Ma. Cr., revised the manuscript, F.G. and L.A. designed the work. S.N., L.A. and F.G. wrote the first draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Negri, S., Pietrolucci, F., Andreatta, S. et al. Bioprospecting of Artemisia genus: from artemisinin to other potentially bioactive compounds. Sci Rep 14, 4791 (2024). https://doi.org/10.1038/s41598-024-55128-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55128-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.